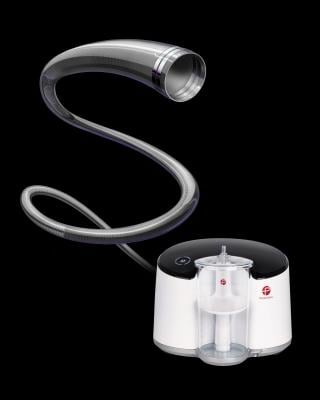
July 25, 2019 — Penumbra announced U.S. commercial availability of the Penumbra System’s most advanced technology, the Penumbra Jet 7 Reperfusion Catheter with Xtra Flex technology, at the Society of NeuroInterventional Surgery (SNIS) 16th Annual Meeting, July 22-25 in Miami. Penumbra Jet 7 with Xtra Flex technology is used with the Penumbra Engine in the fully integrated Penumbra System – an aspiration-based mechanical thrombectomy system that enables physicians to extract thrombus in acute ischemic stroke patients.
The Penumbra System is a fully integrated system designed specifically for mechanical thrombectomy by aspiration that first received 510(k) clearance by the U.S. Food & Drug Administration (FDA) in December 2007. It enables physicians to use aspiration, which acts like a minimally invasive “vacuum” inside the artery to remove stroke-causing blood clots from the brain safely and effectively. It is intended for use in the revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusion.
The Penumbra Jet 7 Reperfusion Catheter with Xtra Flex technology is the latest advancement in the Penumbra System Jet – Engine family of products, which was launched last September. As the eighth-generation reperfusion catheter for proximal large vessel occlusions, the catheter is designed for advanced trackability and navigation, and maintains the large 0.072-inch lumen. Penumbra Jet 7 with Xtra Flex technology together with the power of the Penumbra Engine are designed to maximize thrombus removal force.
Initial clinical experience with the new technology was shared in the Industry Technology Luncheon Symposium by Donald F. Frei, M.D., director of neuro-interventional surgery, Swedish Medical Center, Englewood, Colo.
For more information: www.penumbrainc.com


 November 14, 2025
November 14, 2025 









