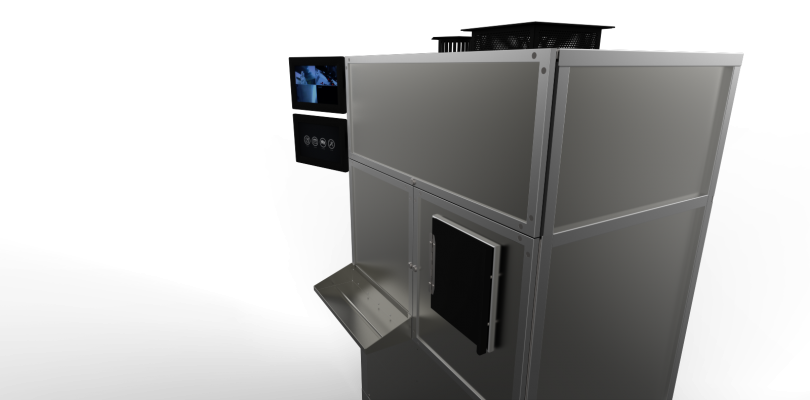
May 16, 2013 — Positron Corp. announced the release of the PosiRx 3000-Series, its latest pharmacy automation systems. The PosiRx 3000-Series are the first systems to automate and encompass the complete compounding process, from generator elution to dose distribution, of multiple diagnostic single photon emission computed tomography (SPECT) agents in an environment engineered to be ISO Class 5 and USP 797 compliant. Designed for facilities dispensing as many as 300 patient-specific doses per day, the PosiRx 3000-Series will benefit providers and patients by enabling unit dose radiopharmaceuticals to be prepared more cost effectively and accurately than previously possible.
The PosiRx line of automation systems features DoseLink, Positron's state of the art proprietary software. Developed for precision and customer ease of use, DoseLink maximizes the functionality and efficiency of operating the PosiRx system through a robust and intuitive interface that can be operated from a variety of interfaces. Whether accessed from a desktop, tablet or smartphone, DoseLink empowers the user to control drug preparation and plan daily production, as well as store and retrieve data in a secure environment. Additionally, the operator may interact with Positron support personnel for assistance with any task in real time, across any platform.
"The release of the PosiRx 3000-Series is a true milestone that marks the next phase in nuclear pharmacy automation," stated Charles Conroy, RPh, MBA, Positron's chief operating officer. "For over 20 years, the nuclear pharmacy community has been seeking an automation solution that can efficiently and accurately prepare patient specific unit doses. Through the ability to compound a wide selection of radiopharmaceuticals, coupled with the system's speed and efficiency, the 3000-Series is that solution and stands in a class by itself. By powering the system with DoseLink, PosiRx users will benefit from the unprecedented flexibility and control to operate across all types of devices and a variety of platforms. By controlling the preparation process from any place at any time, our customers will see significant revenue enhancement from increased patient throughput and a decrease in cost of goods."
To support commercialization efforts, Positron has launched a dedicated website for the PosiRx portfolio of products. The new website is part of Positron's plan to further develop its corporate identity and is designed to help distinguish between the company's cardiac positron emission tomography (PET) business and high tech automation products. By highlighting innovative features and further expanding its capabilities, the site allows visitors to gain a comprehensive understanding of the PosiRx System.
Conroy added, "The PosiRx system with DoseLink software provides a solid platform for growth into other diagnostic areas, additional pharmaceutical classes and new markets. The dedicated website will be very important as we work with our partners to expand Positron's offerings."
For more information: www.positron.com


 August 17, 2023
August 17, 2023 








