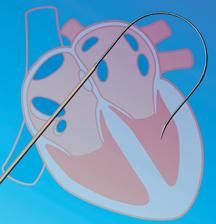
December 4, 2008 - Pressure Products Inc. said this week the FDA granted market clearance to distribute the SafeSept transseptal guide wire, to be used in conjunction with a standard Brockenbrough transseptal needle when performing a transseptal crossing in the setting of atrial fibrillation and other invasive and electrophysiological atrial bi-chamber cardiology procedures.
The company said the device is the first micro-wire accessory for transseptal access and makes a difficult procedure less stressful and enhances patient safety. The SafeSept Transseptal Guidewire is designed to easily perforate the interatrial septum, immediately become atraumatic in the left atrium, enable fluoroscopic confirmation in the pulmonary veins, and allow safer over-the-wire advancement of the transseptal needle, dilator and introducer sheath into the left atrium.
For more information: www.pressure-products.com


 July 21, 2025
July 21, 2025 









