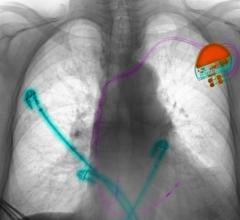
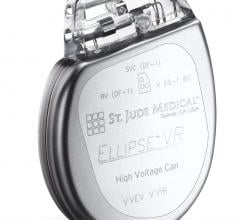
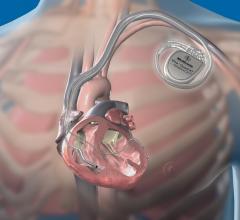
St. Jude Medical said recently the FDA cleared its new delivery tools designed to give physicians greater control and precision when placing cardiac pacing leads. The CPS Duo stylet and guide wire system, the PCS Courier guide wire and the Mond stylet are all tools designed to place leads in precise positions that can be difficult to access.
The CPS Duo system is a combination of a hollow stylet and compatible guide wire. The guide wire aims to provide flexibility and support while the stylet provides stiffness needed to advance the lead and manipulate its tip. Together, the products provide greater maneuverability and control of the left-ventricular lead than either tool could supply alone, said the company.
|

 February 24, 2023
February 24, 2023