February 15, 2018 — The U.S. Food and Drug Administration (FDA) has expanded the indication for Stryker's Trevo Retriever as a front-line treatment for patients experiencing acute ischemic stroke up to 24 hours from symptom onset, increasing the treatment window by 18 hours. The expanded indication of Stryker's clot-removal device is in line with the recently updated treatment guidelines from the American Heart Association (AHA) and American Stroke Association (ASA), and has the potential to reduce disability and improve quality of life for tens of thousands of additional stroke patients each year.
"The 24-hour indication opens the treatment window to patients whose stroke would previously have progressed until all the brain tissue surrounding the affected arteries was dead, leaving them with a life of significant disabilities," said Raul Nogueira, M.D., of Grady Memorial Hospital and Emory University, Atlanta. "These patients now have a much better chance for an independent life without disability."
Stryker's Trevo Retriever is the first and only thrombectomy device to receive FDA clearance to significantly reduce disability in patients up to 24 hours from symptom onset, according to the company. Until now, mechanical thrombectomy devices like the Trevo Retriever were only cleared for use up to six hours from symptom onset – leaving a significant population of stroke patients ineligible to receive treatment. However, recent randomized clinical data from the Stryker-sponsored DAWN Trial shows that patients presenting in the six- to 24-hour window who meet specific imaging criteria and are treated with the Trevo Retriever are almost four times as likely to be functionally independent at 90 days post-stroke, compared to those treated with medical management alone.1
"FDA clearance of the Trevo Retriever has effectively quadrupled the treatment window from zero-six hours to zero-24 hours from time last seen well. Nonetheless, every minute still matters because in each individual patient, the earlier the blood flow is restored to the brain, the lower the resultant burden of permanent brain damage. Therefore, it is still imperative that all caregivers in the stroke pathway – from EMS to emergency room physicians to stroke neurologists to interventionalists – continue to deliver the fastest and most workflow-efficient treatment of stroke," said Tudor Jovin, M.D., University of Pittsburgh Medical Center.
The Trevo Retriever was used exclusively in the DAWN Trial and was included in the DEFUSE 3 study – producing the largest cohort of data for a single device across the two late window stroke trials that led to the recent updates to the AHA/ASA guidelines. Based on the depth of clinical evidence, stent retrievers like the Trevo Retriever are recommended as the standard of care for stroke patients experiencing a large artery blockage by the American Heart Associations' Stroke Guidelines.2
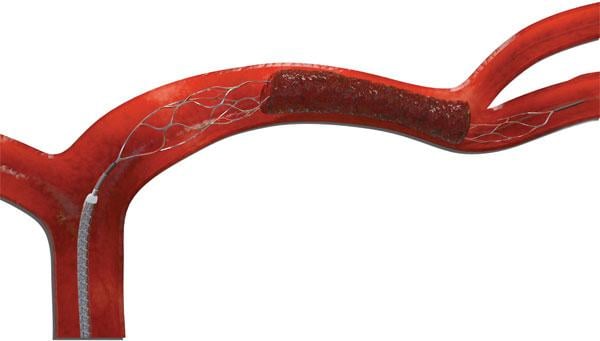
The Trevo Retriever is a tiny stent-shaped medical device that is attached to a thin wire. In a minimally invasive procedure that utilizes X-ray, the physician navigates the retriever from the femoral artery, which is located in the upper leg, to the blocked blood artery in the brain. The retriever is designed to ensnare the blood clot and remove it from the body. Originally cleared by the FDA in 2012, the Trevo Retriever has been used in thousands of patients worldwide.
For more information: www.stryker.com
References
2. Powers, W.J. et. al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. January 30, 2018. stroke.ahajournals.org


 November 14, 2025
November 14, 2025 









