March 2, 2017 — Teleflex Inc. has announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U.S. commercial launch of the TrapLiner Catheter.
The TrapLiner Catheter was developed by Vascular Solutions Inc., which was acquired by Teleflex on Feb. 17, 2017.
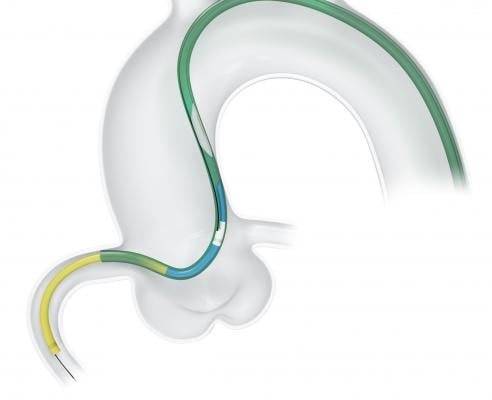
The TrapLiner Catheter is similar in design to Vascular Solutions’ popular GuideLiner Guide Extension Catheter, with the added feature of an integrated balloon for trapping a standard 0.014-inch guidewire within a guide catheter. The TrapLiner Catheter can be used as an alternative method to the trapping technique that requires the use of a percutaneous transluminal coronary angioplasty (PTCA) balloon to exchange an existing over-the-wire catheter while maintaining guidewire position. The technique of guidewire trapping for catheter exchange is most commonly performed in complex interventional procedures. The device is offered in three different sizes: 6, 7, and 8 Fr.
“The TrapLiner is a one-of-a-kind product that combines two devices we had previously deployed independently during challenging cases into a single tool that enables the most complicated interventional procedures to be done more efficiently,” said Dimitri Karmpaliotis, M.D., Ph.D., director of CTO, complex and high-risk angioplasty at Columbia University Medical Center, who was the first to use the device in the United States. “The primary clinical use for the TrapLiner is during cases in which over-the-wire microcatheters are required to cross calcified lesions, navigate bifurcations or cross tortuous anatomy. During these procedures, the TrapLiner not only provides added backup support and deep-seating for the guide catheter, but also allows the operator to maintain guidewire positioning when exchanging the microcatheter. The distinct two-in-one capability of this device is highly desirable, as it greatly enhances the efficiency of complex procedures.”
The TrapLiner Catheter is intended for use in conjunction with guide catheters to access discrete regions of the coronary and/or peripheral vasculature, to facilitate placement of interventional devices, and to facilitate the exchange of an interventional device while maintaining the position of the guidewire within the vasculature.
For more information: www.teleflex.com


 November 14, 2025
November 14, 2025 









