Taking anti-platelet medication longer than current recommendations may lower heart attack or death risks for patients ...
The FDA was notified on Dec. 2 that Pfizer will suspend a large, Phase 3 trial evaluating the investigational ...
The FDA will revisit a device tomorrow that it cleared for U.S. sale three years ago — drug-eluting stents will be the ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The American Heart Association has published a report demonstrating that adolescent boys have a significantly increased ...
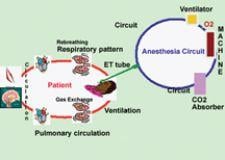
Pulse oximetry, which directly monitors the patient's oxygenation, came into its own as standard medical practice 20 ...
The Society for Cardiac Angiography and Interventions (SCAI) has extended the abstract deadline for its 30th Annual ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
One of the major dilemmas faced by clinicians in the Intensive Care Unit (ICU) is the determination of volume status and ...
Toronto-based Cedara Software, a Merge Healthcare company and an independent developer of medical software technologies ...
Recent downplay of the media’s attention to DES-related thrombosis during both the TCT and AHA conferences this fall may ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
CV Therapeutics has announced it will file a new drug application to the FDA for its regadenoson agent in mid-2007 ...
AngioDynamics, Inc. has announced FDA 510(k) clearance for Oncobionic, Inc. to market Irreversible Electroporation (IRE) ...
Possis Medical, Inc. has received clearance from the FDA to market its AngioJet Xpeedior catheter to remove blood clots (thrombus) from upper- and lower-extremity peripheral veins. Used with the company’s AngioJet System, Xpeedior is the only device cleared for thrombectomy in peripheral veins.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Two novel polymer-free drug-eluting stent coating technologies tested in a comparative animal study has concluded with ...
Magnets may interfere with the operation of pacemakers and implantable cardioverter defibrillators (ICDs), according to a study published in the December 2006 edition of “Heart Rhythm.”
An implantable technology to control high blood pressure in patients not responding to available medicines has ...

 December 06, 2006
December 06, 2006





