The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, said to be the world’s first high definition CT scanner that ...
The FDA granted 510(k) clearance for the AIGISRX CRMD Anti-Bacterial Envelope by Tyrxpharma, a cardiac rhythm medical ...
May 13, 2008 – The FDA approved changes to the U.S. product label for DEFINITY Vial For (Perflutren Lipid Microsphere) ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Abbott Vascular’s HI-TORQUE WHISPER guidewire is designed to offer a higher level of support to facilitate device ...
The Edwards Lifesciences unveiled a new software upgrade that allows greater flexibility to trend and analyze the ...
May 13, 2008 - Edwards Lifesciences unveiled a new software upgrade that allows greater flexibility to trend and analyze ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The FDA cleared for marketing a new automated version of diaDexus’ proprietary PLAC Test, an automated immunoassay designed to run on common existing laboratory equipment, including clinical chemistry analyzers from Hitachi, Roche/Hitachi and Olympus, expanding the number of clinical labs and physician offices that are able to offer the PLAC Test.
TomTec Imaging Systems recently announced that its new CardioArena, the network and imaging solution for cardiology ...
May 9, 2008 – Philips started shipping the upgrade Xcelera R2.2, a new version of the Xcelera multimodality cardiology ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 9, 2008 – The Centers for Medicare and Medicaid Services (CMS) issued its final National Coverage Decision (NCD) to ...
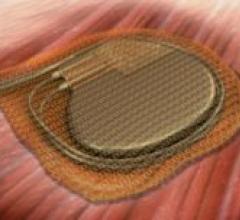
May 9, 2008 - The FDA cleared Boston Scientific's ALTRUA family of pacemakers, a day after the European approval of ...
At the 2008 American Association of Critical-Care Nurses National Teaching Institute, Holtech Medical displayed its new FoleyManometer LV to help monitor intra-abdominal pressure (IAP).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
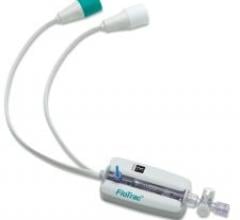
May 9, 2008 – The FDA approved Covidien’s contrast delivery system with radio-frequency identification (RFID) technology, integrating the technology to create a system that is designed to enhance patient safety by reducing the risk of medical errors in radiology departments.
May 9, 2008 – Philips launched the new Xcelera R2.2, the latest version of its Xcelera multimodality cardiology image management, analysis and reporting solution, offering improved support for imaging studies and data from key cardiac subspecialties including cardiac catheterization and cardiovascular ultrasound, and additionally supports nuclear cardiology, cardiac CT, cardiac MRI and electroph
Medical gas and vacuum products maker Ohio Medical Corp. released its new Push-To-Set Digital vacuum regulators product ...


 May 12, 2008
May 12, 2008