At the 2008 American Association of Critical-Care Nurses National Teaching Institute, Holtech Medical displayed its new FoleyManometer LV to help monitor intra-abdominal pressure (IAP).
May 9, 2008 – The FDA cleared the Strada Carotid Guiding Sheath, a flexible tube for physicians to deliver balloon ...
May 9, 2008 – Philips launched the new Xcelera R2.2, the latest version of its Xcelera multimodality cardiology image management, analysis and reporting solution, offering improved support for imaging studies and data from key cardiac subspecialties including cardiac catheterization and cardiovascular ultrasound, and additionally supports nuclear cardiology, cardiac CT, cardiac MRI and electroph
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
May 8, 2008 – The first human procedure using the Volcano OCT imaging catheter, which uses optical coherence tomography (OCT) designed to enhance the diagnosis and treatment of coronary and peripheral vascular disease, was performed by Patrick Serruys, M.D., at the Thoraxcenter, in Rotterdam, Netherlands.
A safety engineered short-term peripheral intravenous (IV) catheter, the VantageCath, is designed to reduce exposure to ...
May 7, 2008 – Lantheus Medical Imaging initiated CaRES (Contrast Echocardiography REgistry for Safety Surveillance), a ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
CorMatrix ECM by CorMatrix Cardiovascular is for Cardiac Tissue Repair, which utilizes the company’s proprietary ...
May 7, 2008 - The FDA has cleared for marketing a new safety engineered short-term peripheral intravenous (IV) catheter ...
May 7, 2008 - GE Healthcare signed a nonexclusive agreement with Merck and Co. to provide spin signal technology (SST) ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 6, 2008 - Cordis Corp. U.S. launch of the SLEEK and SAVVY Long PTA Balloon Dilatation Catheters, representing the ...
May 5, 2008 - HeartWare Ltd. received conditional approval from the FDA of an Investigational Device Exemption (IDE) for ...
May 6, 2008 - Vascular Insights received 510(k) clearance from the FDA to market its ClariVein infusion catheter for infusion of physician-specified agents in the peripheral vasculature. ClariVein is a percutaneous, 2 2/3 Fr (0.035-inch) catheter, containing a rotating wire driven by a motor, that enhances fluid dispersion in the treatment area.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 6, 2008 - Cordis Corp.'s U.S. launch of the SLEEK and SAVVY Long PTA Balloon Dilatation Catheters represents the ...
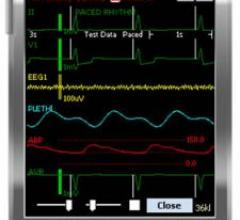
May 6, 2008 - Philips' IntelliVue mobile viewer application gives physicians access to patient information from anywhere within the hospital by providing patient data and alerts through a personal digital assistant (PDA) device.
May 6, 2008 - Philips' IntelliVue mobile viewer application gives physicians access to patient information from anywhere within the hospital by providing patient data and alerts through a personal digital assistant (PDA) device.

 May 08, 2008
May 08, 2008