Sunshine Heart Inc. announced its collaboration with Minnetronix for a transcutaneous energy transfer (TET) system to power the company's fully-implantable C-Pulse Heart Assist System under development. The agreement leverages Minnetronix's existing TET technology and includes milestone payments due to Minnetronix prior to U.S. regulatory approval for the fully-implantable C-Pulse system.
In a paper e-published in Catheterization and Cardiovascular Interventions, the Society for Cardiovascular Angiography and Interventions (SCAI) examined the current state of medical simulation in interventional cardiology and issued recommendations for expanding and standardizing the use of the lifelike training technology by practicing interventional cardiologists and fellows-in-training
Cardiovascular Systems Inc. (CSI) announced that it has received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) to market its Diamondback 360 Coronary Orbital Atherectomy System (OAS) as a treatment for severely calcified coronary arteries.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 23, 2013 — Vascular Interventional Advances (VIVA) Physicians, a not-for-profit organization dedicated to advancing the field of vascular medicine and intervention through education and research, announced 15 trial results at VIVA 13. The trial results offered new information on advances in the treatment of vascular diseases.
Bracco Diagnostics Inc. announced the availability of syringe packs with spikes, to be used when loading contrast and saline for delivery with power injectors in computed tomography (CT) suites. Bracco syringe packs are specifically designed for use with the EmpowerCTA and Empower CT contrast delivery systems, offering greater flexibility in contrast and saline preparation.
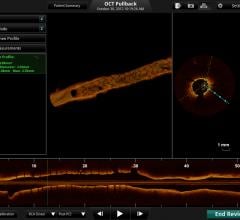
St. Jude Medical Inc. announced the U.S. Food and Drug Administration (FDA) approval and launch of its Ilumien Optis PCI optimization system, a new technology designed to provide physicians with a comprehensive disease assessment tool for treating patients with coronary artery disease (CAD). The system will be on display for the first time in the United States during the 2013 Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium in San Francisco.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
October 22, 2013 — AtheroMed, a developer of catheter technologies for treating peripheral artery disease (PAD), announced results from its Endovascular Atherectomy Safety and Effectiveness (EASE) study during a late-breaking clinical trials session at the Vascular Interventional Advances (VIVA) 2013 conference in Las Vegas. Stephen Williams, M.D., director of the Vascular Medicine Center, Johns Hopkins University, presented the results of the study, which demonstrate the safety and effectiveness of the Phoenix atherectomy system in treating PAD.
A 56-year-old man who had a heart attack survived and is recovering at home after receiving two hours and forty-five minutes of cardiopulmonary resuscitation (CPR). The length of CPR time is believed to be among the longest on record and was made possible because of a mechanical chest compression machine called the Lucas device.
AngioScore Inc., a developer of angioplasty catheters for use in the treatment of cardiovascular disease, announced that preliminary data from the first-in-human (FIH) study (“Patent-C”) of the drug-coated AngioSculpt Scoring Balloon Catheter will be presented at the Transcatheter Cardiovascular Therapeutics (TCT) Conference in San Francisco the week of Oct. 28.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Nuclear Imaging Services LLC (NIS) has completed the first U.S.-based ECAT Scintron installation into an outpatient cardiology facility. Gulf Coast Cardiology Group in Port Arthur, Texas, is the first to upgrade its Siemens ECAT system to Medical Imaging Electronics' (MiE) ECAT Scintron.

Medtronic Inc. announced the first implants in the CoreValve Evolut R clinical study, which will evaluate the safety and effectiveness of the CoreValve Evolut R recapturable system. This recapture-enabled valve and delivery system is designed to advance deliverability and valve performance while providing the option to recapture and reposition the CoreValve Evolut R valve during deployment, if needed, while performing transcatheter aortic valve implantation (TAVI).
Epsilon Imaging Inc., a visualization and analysis software provider, announced the launch of its newest application specifically designed for right ventricle (RV) assessment. EchoInsight computer-aided visualization and analysis offers quick and intuitive strain imaging along with automated cardiac function measurements for enhanced confidence and workflow in echo interpretation.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Pie Medical Imaging B.V. (PMI) announced that they have signed an agreement with the Cardiovascular Research Foundation (CRF) to begin a close collaboration. Under this agreement, the CRF Clinical Trials Center will use PMI’s solutions in their Angiographic Core Laboratory for clinical research and multicenter trials. CRF will also support further development of PMI’s new products for analysis and visualization of medical images.
Medtronic Inc. announced the U.S. launch of the Export Advance aspiration catheter, a U.S. Food and Drug Administration (FDA)-cleared catheter that features a pre-loaded stylet that increases deliverability and kink resistance while traversing the vasculature to reach the aspiration site.
Bard Peripheral Vascular has sent an urgent Class I medical device recall notification letter informing customers of the problems of the LifeStent Solo vascular stent and the actions customers should take. The LifeStent Solo vascular stent is an implantable self-expanding stent and delivery system used to improve the superficial femoral artery (SFA) luminal diameter in the treatment of atherosclerotic lesions.

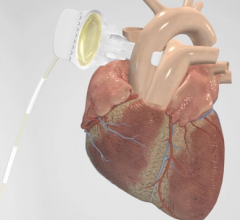
 October 23, 2013
October 23, 2013