The Pinnacle R/O II HiFlo Introducer Sheath is a 4-cm stiff introducer sheath that features a smooth transition that ...
MasterScreen CPX is reportedly a complete exercise test that includes a stress ECG option. Its digital TripleV volume ...
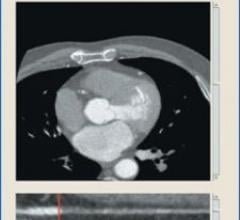
Voxar 3-D 6.1 visualization and analysis software incorporates a suite of clinical applications for CT cardiac analysis ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Human Patient Simulator (HPS) – a computer-model-driven, full-sized mannequin – delivers experience in true-to-life ...
The Avanta Fluid Management Injection System is intended to help cardiovascular healthcare professionals improve patient ...
Cholestech Corp., provider of point-of-care health management solutions for chronic disease, and Life Line Screening ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The intra-aortic balloon (IAB) is an minimally invasive technology designed to enhance blood flow to the heart and other ...
ScImage’s latest additions to its Web-based cardiology viewing suite, PicomEnterprise, reportedly allowing gated SPECT ...
The COR Analyzer I by Rcadia Medical Imaging assists screening of triage patients for coronary artery disease. The ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
When used in conjunction with the ev3 embolic protection device, ev3’s PROTÉGÉ RX Carotid Stent has been FDA cleared for ...
The AtriCure Ablation and Sensing Unit (ASU) is a device that makes linear and transmural lesions in a matter of seconds ...
March 19, 2007 — Onset Medical Corp. has announced it has received FDA clearance to begin marketing the SoloPath ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 19, 2007 — AT&T Inc. has announced an agreement wherein it will upgrade the conference platform used by Medical ...
March 19, 2007 — Medtronic Inc. has launched its U.S. pivotal clinical trial for the Cardioblate Surgical Ablation ...
March 19, 2007 — “Health IT Strategist” reports that San Francisco-based Practice Fusion is offering its Web-based ...


 March 20, 2007
March 20, 2007