June 13, 2012 — Positron Corp. announced completion of its first strontium validation effort in May 2012, when Manhattan Isotope Technology (MIT), a Positron subsidiary, successfully processed strontium.
As the nation’s leading provider of maternal-fetal, newborn and pediatric subspecialty physician services, Pediatrix Medical Group says streamlining the transfer of images and reports is a priority. Seeking a faster, more reliable method of exchanging files than on CD, the group adopted a cloud-based medical information exchange.
Best Cyclotron Systems is unveiling five new cyclotrons for isotope research and production at the Society of Nuclear Medicine (SNM) 2012 Annual Meeting on June 9-12 in Miami Beach, Fla.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
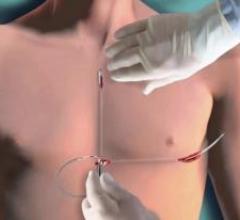
Boston Scientific Corporation has closed its acquisition of Cameron Health and, as a result, added to its product portfolio the world's first and only commercially available subcutaneous implantable cardioverter defibrillator, called the S-ICD System.
June 11, 2012 — Boehringer Ingelheim Pharmaceuticals Inc. announced that the Pradaxa (dabigatran etexilate mesylate) capsules prescribing information has been updated to affirm that "Pradaxa 150 mg twice daily was superior in reducing ischemic and hemorrhagic strokes relative to warfarin."
June 8, 2012 — Bayer HealthCare announced the formation of a strategic partnership with Radimetrics Inc. that will offer U.S. healthcare professionals the industry’s first computed tomography (CT) dose management software portfolio to include radiation and contrast dose tracking. Through the agreement, Bayer will distribute the Radimetrics eXposure Radiation Dose Management (RDM) software in addition to Bayer’s existing contrast-focused dose management solution.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
June 11, 2012 — Critical Diagnostics, a U.S.-based biomarker company focused on cardiovascular diseases, and Health Diagnostic Laboratory Inc. (HDL) announced an agreement under which HDL will soon offer ST2 testing services based on Critical Diagnostics' Presage ST2 Assay.
June 11, 2012 — Calgary Scientific Inc. has received clearance from the U.S. Food and Drug Administration (FDA) to market its ResolutionMD Web 2.9 Vessel Analysis module for post-processing diagnostic review and analysis of patient computed tomography (CT) and magnetic resonance imaging (MRI) scans.
June 11, 2012 — An estimated 85.3 million computed tomography (CT) procedures were performed in 8,465 hospital and non-hospital sites in 2011 in the United States, representing an annual growth rate of 4 percent compared to 2010, when an estimated 81.9 million procedures were performed, according to a new survey of CT sites in the U.S. by IMV Medical Information Division.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

June 8, 2012 — Children and young adults scanned multiple times by computed tomography (CT) have a small increased risk of leukemia and brain tumors in the decade following their first scan. These findings are from a study of more than 175,000 children and young adults that was led by researchers at the National Cancer Institute (NCI), part of the National Institutes of Health, and at the Institute of Health and Society, Newcastle University, England.
June 8, 2012 — Bayer Radiology and Interventional has announced the introduction of the Certegra Workstation, an informatics-driven, touch-screen hub that enables time savings and improved patient care through a suite of CT (computed tomography) Contrast Dose Management applications and a new P3T 2.0 operating environment.
June 8, 2012 — The U.S. Food and Drug Administration (FDA) has approved revised product labeling for Boston Scientific Corp.’s Incepta, Energen, Punctua, Cognis and Teligen implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds), to reflect increased longevity projections for these devices. The longevity projections are based on data submitted to the FDA and vary for each device dependent on the model type and settings.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
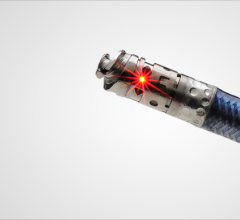
June 8, 2012 — Austin Heart announced its participation in CONNECT II, a global clinical trial that gives physicians access to a new imaging technology tool to fight peripheral arterial disease (PAD). The technology, called Ocelot, helps to eliminate the need for bypass surgeries and/or amputations in patients with the disease.
June 8, 2012 — Medtronic Inc. announced the U.S. launch of the Endurant II AAA Stent Graft System, which recently received approval from the U.S. Food and Drug Administration (FDA) for the minimally invasive treatment of abdominal aortic aneurysms through endovascular repair (EVAR), an alternative to major surgery.
June 8, 2012 — LifeWatch AG, a wireless cardiac monitoring service and home sleep test provider in the United States, announced the launch of the world’s first-of-its-kind healthcare solution smartphone, allowing consumers self-operation of a wide range of highly valuable embedded medical sensors as well as wellness-related applications and cloud-based services. With this new technology LifeWatch will be able to expand outside its core markets, targeting new customer segments, service offerings and geographies. A comprehensive presentation and live demonstration of the solution will be given in Zurich on June 27, 2012.

 June 13, 2012
June 13, 2012