Boston Scientific Corp. received CE marking for the Rebel Platinum Chromium Coronary Stent System, the company's latest generation bare metal stent for the treatment of coronary artery disease (CAD).
Janssen Research & Development LLC (Janssen) announced the U.S. Food and Drug Administration (FDA) issued complete response letters (CRLs) regarding supplemental New Drug Applications (sNDAs) for the use of rivaroxaban, an oral anticoagulant, to reduce the risk of secondary cardiovascular events — defined as heart attack, stroke or death — in patients with acute coronary syndrome (ACS) and to reduce the risk of stent thrombosis in the same population, in combination with standard antiplatelet therapy.
Privately held On-X Life Technologies Inc. (On-X LTI) launched its European marketing campaign for the On-X Plus 1.5 Aortic Heart Valve in concert with its Great Britain distributor Vascutek Corp. at the Annual Meeting & Cardiothoracic Forum of the Society for Cardiothoracic Surgery in Great Britain & Ireland, March 10-12, Edinburgh, Scotland.
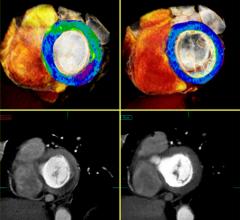
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Micell Technologies Inc. announced that imaging and clinical results from the DESSOLVE I and DESSOLVE II trials of its MiStent Sirolimus Eluting Absorbable Polymer Coronary Stent System (MiStent SES) were presented at the 25th Annual Transcatheter Cardiovascular Therapeutics (TCT) Conference held in San Francisco, Oct. 27 to Nov. 1, 2013.
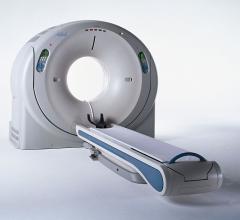
Houston Healthcare is putting patient safety first by offering low dose computed tomography (CT) exams with the industry’s best solutions and a comprehensive dose management approach from Toshiba America Medical Systems Inc.
iRhythm Technologies Inc., a healthcare information services company, announced that new study data support the use of its ZIO Service to help identify underlying cardiac arrhythmias in patients who have had a stroke or transient ischemic attack (TIA).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The U.S. Food and Drug Administration (FDA) cleared Toshiba’s CT Myocardial Perfusion capability. Available on Toshiba’s Aquilion One and Aquilion OneVison Edition CT systems, Myocardial Perfusion allows clinicians to visualize myocardial ischemia with CT, providing a clinical and operational solution to make work flow.
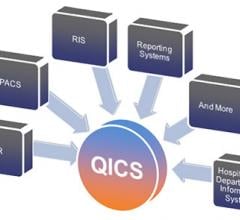
McKesson announced a cardiology solution called Qualitative Intelligence and Communication System (QICS). QICS for cardiology helps organizations automate and manage cardiology workflows to promote efficiency and effectiveness. The solution offers integrated workflows and results communications management within the cardiovascular information system (CVIS).
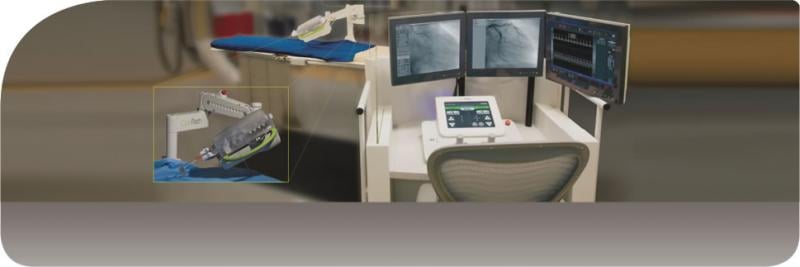
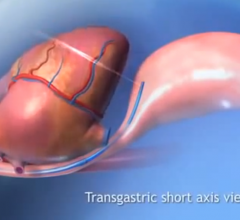
For patients living in rural areas, life-saving procedures such as an angioplasty may only be available at a facility more than 200 miles away. To bridge this distance, Corindus Vascular Robotics is partnering with Sanford Health and The Leona M. and Harry B. Helmsley Charitable Trust to launch a feasibility study for a remote robotic systems cath lab telecardiology program.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Countries across the globe are struggling to combat issues such as escalating healthcare costs, poor or inconsistent quality of healthcare, rapid expansion in healthcare insurance and changing healthcare reform mandates. Growing consumerism, globalization, changing demographics and lifestyles, and growing incidences of diseases that are expensive to treat further exacerbate these. Resolving these issues is a daunting task faced by healthcare stakeholders, highlighting the need for proactive, collaborative and systemic models. Several initiatives and healthcare reforms have been developed in order to support the adoption and implementation of clinical decision support software (CDSS) solutions.
The U.S. Food and Drug Administration (FDA) cleared Cardiovascular Systems Inc.’s Diamondback 360 60 cm Peripheral Orbital Atherectomy Systems (OAS) for the treatment of peripheral arterial disease (PAD).

Guerbet announced a worldwide launch of FlowSens, a contrast agent injection solution used in X-ray medical examinations. FlowSens received CE marking in the first quarter of 2014. It will initially be marketed in Europe (first in Belgium, France, Germany and Switzerland), then gradually in other regions of the world.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Marty Greenfield lives with crushing pain every day due to angina. He has suffered a heart attack, and a coronary bypass procedure and angioplasty have provided little relief. His doctor referred him to University of California, Los Angeles to be considered for a heart transplant.
After a heart attack, there is often permanent damage to a portion of the heart. This happens, in part, because cardiac muscle cells are terminally differentiated and cannot proliferate after blood flow is blocked off to the heart. This partial healing can be attributed to heart disease being one of the leading causes of death. What if the cells could be stimulated to divide and the heart could be induced to repair itself? This was the question posed by George Washington University researcher Scott Shapiro, M.D., Ph.D., and his co-authors. They found cardiac regeneration may be a possibility with gene therapy.
ImaCor Inc., the developer of hemodynamic transesophageal echocardiography (hTEE) technology, announced four academic hTEE presentations. Physicians are publicly recognizing hTEE as a tool for managing complex ICU patients.


 March 13, 2014
March 13, 2014