Each year, deep vein thrombosis (DVT) leads to hospitalization for approximately 250,000 Americans, and often leads to serious — and even fatal —complications. Fortunately, at the California Institute for Deep Venous Thrombosis, those suffering from the effects of thrombosis will find an effective DVT treatment from Farshad Malekmehr.
Surgical aortic valve replacement generally improves patients’ symptoms and prolongs survival. However, the perceived risk of surgical aortic valve replacement in patients over 80 may result in surgery being denied or a recommendation for alternative therapy. Investigators at the Mayo Clinic challenge the way these patients have been managed. They report that repeat sternotomy in patients over 80 who have previously had coronary bypass graft surgery can be done with low risk. T
July 15, 2014 — Micell Technologies Inc. announced the United States Patent and Trademark Office (USPTO) has issued a patent related to Micell's technology for enhancing the performance of medical devices with precise drug-delivery coatings.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
July 15, 2014 — Chelation treatments reduce cardiovascular events, such as heart attacks, and death in patients with diabetes but not in those who did not have diabetes, according to analyses of data from the National Institutes of Health (NIH)-funded Trial to Assess Chelation Therapy (TACT). However, researchers say more studies are needed before it is known whether this promising finding leads to a treatment option.

July 15 2014 — Researchers at the University of California, San Diego School of Medicine have documented the safety benefits of aortic stent grafts inserted during minimally invasive surgery to repair abdominal aortic aneurysms in a study published July 9 in JAMA Surgery.
July 15, 2014 — Telemed Solutions has reached an agreement with GlobalMedia Group LLC to become the exclusive global distributor for the TM-12, a PC-based wireless resting 12-lead ECG (electrocardiography) system. GlobalMed also will act as the exclusive master distributor for order fulfillment to Telemed Solutions’ existing distributors, resellers and customers.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Tony Parris has worked for banks and electronics companies and has even driven an 18-wheel truck 995 miles a day for more than nine years as part of his working career. The 57-year-old Anniston, Ala., native finally went into business for himself when he opened his own hardware store in Alexandria, Ala., in 2010.
Objective Medical Systems LLC announced that its Electronic Health Record (EHR) system has earned Meaningful Use Stage 2 Certification from the Office of the National Coordinator for Health Information Technology.
DC Devices Inc. has closed a $34 million Series D financing round. Accelmed led the round with contributions from existing investors Third Rock Ventures, General Catalyst Partners and Lumira Capital, as well as a new undisclosed strategic investor. Funds will be used to complete clinical evaluation of the world’s first transcatheter device for the treatment of diastolic heart failure (DHF), a $5 billion global market opportunity.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
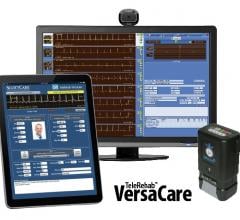
The ScottCare Corp. announced the newest version of its multi-parameter telemetry solution, TeleRehab VersaCare 2.1, which is the industry’s first Windows 7-based cardiopulmonary rehab telemetry system and includes direct integration with the American Association of Cardiovascular and Pulmonary Rehabilitation's (AACVPR) Outpatient Cardiac Rehabilitation Data Registry.
July 11, 2014 — Cordis Corp. announced the launch of its Saber PTA (percutaneous transluminal angioplasty) dilatation catheter for the treatment of patients with peripheral arterial disease (PAD).
July 11, 2014 — On July 3, the Centers for Medicare & Medicaid Services (CMS) issued a proposed rule that would update payment policies and payment rates for services furnished under the Medicare Physician Fee Schedule (PFS) on or after Jan. 1, 2015.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 11, 2014 — Toshiba America Medical Systems introduced its newest M-Power software version for the Vantage Titan 1.5T magnetic resonance (MR) system and a new software version for its Vantage Titan 3T MR. Toshiba will also offer the new software versions with enhanced automated tools at no charge to existing Titan customers with previous M-Power software versions that have a current service contract with Toshiba.
July 10, 2014 — 480 Biomedical announced it has been awarded Phase II funding from the National Heart, Lung, and Blood Institute (NHLBI) to continue the development of a bioresorbable, self-expanding scaffold to treat pediatric pulmonary artery stenosis (PAS).
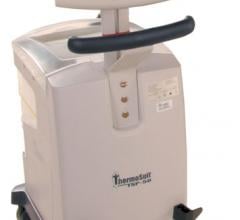
July 10, 2014 — Life Recovery Systems announced the U.S. Food and Drug Administration (FDA) has approved two investigational device exemptions (IDEs) to study the use of the ThermoSuit system in the treatment of heart attack and ischemic stroke. Patients in both trials will be cooled while under sedation, with the intent of reaching a state of therapeutic hypothermia in approximately 30 minutes or less.

 July 15, 2014
July 15, 2014