The FDA cleared for marketing a new automated version of diaDexus’ proprietary PLAC Test, an automated immunoassay designed to run on common existing laboratory equipment, including clinical chemistry analyzers from Hitachi, Roche/Hitachi and Olympus, expanding the number of clinical labs and physician offices that are able to offer the PLAC Test.
TomTec Imaging Systems recently announced that its new CardioArena, the network and imaging solution for cardiology ...
May 13, 2008 – Spectranetics’ excimer laser sheath safely and effectively assists removal of pacing and defibrillator leads, according to the study, “Large, Single-catheter, Single-operator Experience with Transvenous Lead Extraction: Outcomes and Changing Indications,” featured in the April issue of HeartRhythm.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Northeast Monitoring received clearance from the FDA to sell its DR200 Series devices with its automatic atrial ...
Boston Scientific Corp. said the FDA approved three products in its Cardiac Rhythm Management business, including the ...
May 13, 2008 – Echoserve launched an online portal designed to facilitate the research, purchase and sale of diagnostic ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Boston Scientific’s IQ Guidewire is designed to provide the torque response, side branch access support and device ...
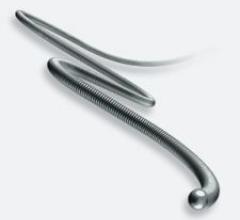
The Promote RF CRT-D and Current RF ICD feature InvisiLink radiofrequency (RF) telemetry for reportedly secure, wireless communication between the implanted device and the programmer, have extended their reach with the new Durata lead.
May 9, 2008 – The Centers for Medicare and Medicaid Services (CMS) issued its final National Coverage Decision (NCD) to ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 9, 2008 - The FDA cleared Boston Scientific's ALTRUA family of pacemakers, a day after the European approval of ...
May 9, 2008 – Philips started shipping the upgrade Xcelera R2.2, a new version of the Xcelera multimodality cardiology ...
May 9, 2008 – The FDA approved Covidien’s contrast delivery system with radio-frequency identification (RFID) technology, integrating the technology to create a system that is designed to enhance patient safety by reducing the risk of medical errors in radiology departments.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 9, 2008 – Philips launched the new Xcelera R2.2, the latest version of its Xcelera multimodality cardiology image management, analysis and reporting solution, offering improved support for imaging studies and data from key cardiac subspecialties including cardiac catheterization and cardiovascular ultrasound, and additionally supports nuclear cardiology, cardiac CT, cardiac MRI and electroph
Medical gas and vacuum products maker Ohio Medical Corp. released its new Push-To-Set Digital vacuum regulators product ...
May 9, 2008 – The FDA cleared the Strada Carotid Guiding Sheath, a flexible tube for physicians to deliver balloon ...

 May 12, 2008
May 12, 2008