Point-of-care blood analysis systems company Abaxis Inc. said the FDA granted waived status for its comprehensive metabolic panel, basic metabolic panel and electrolyte panel, allowing lab-accurate comprehensive health screenings in nearly any environment.
Cardinal Health and HemCon Medical Technologies Inc. in December announced the availability of co-labeled chitosan-based ...

With the progression toward a seamless electronic health records (EHR), seamlessly connecting numerous medical devices ...
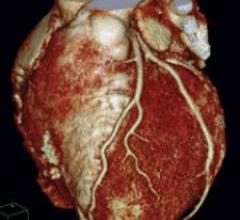
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
February 21, 2008 - Textronics Inc. received FDA clearance to market its textile-based ECG Electrode for use in general ...
The Pro Focus OR ultrasound system is a fully featured OR suite that works as an integrated part of the operating room ...
The Philips 256-slice Brilliance iCT scanner provides 8 centimeters of coverage, covering the entire heart in two scans and including a rotation speed of 0.27 seconds with 120 kw of power.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
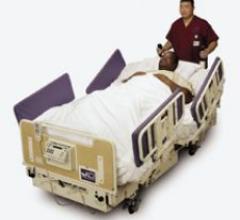
The BariMaxx II Power Drive System is a 1,000-pound capacity expandable side exit bariatric bed with an integrated power ...

Feds Helping Boost Technology in Rural America Via Telemedicine
February 20, 2008 - At HIMSS 2008, Siemens Healthcare will emphasize its image data management solutions for radiology ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Toshiba America Medical Systems Inc. recently introduced its Infinix VF-i/SP X-ray system, a universal cardiovascular ...
The ACUSON X300 works to provide compact, portable color Doppler solutions for adult, pediatric, OR, EP and ...
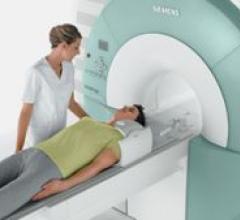
Siemens Medical Solutions received FDA 510(k) clearance for a 1.5T magnetic resonance imaging (MRI) system that is ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

February 2008 - The traditional hospital cleaning methods of wiping down surfaces and mopping floors are now being ...
February 20, 2008 - Philips Medical Systems features at HIMSS 2008 enhancements to its speech recognition solution ...
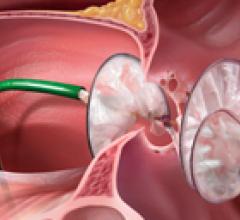
W. L. Gore & Associates (Gore) announced that the FDA granted approval for the use of GORE HELEX Septal Occluder with a modified catheter delivery system indicated for the transcatheter closure of atrial septal defect (ASD), providing a percutaneous ASD closure solution for very young patients.

 February 20, 2008
February 20, 2008