February 10, 2009 - On Jan.
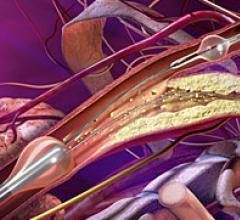
February 10, 2009 - W. L. Gore and Associates today said at the International Congress on Endovascular Interventions XXII the FDA cleared the GORE Flow Reversal System, which minimizes the risk of emboli reaching the brain during carotid artery stenting (CAS).
February 10, 2009 - Four hospitals in western New York state used emergency treatment strategies emphasizing evidence-based therapy and better communication among healthcare providers to reduce heart attack patient deaths by 19 percent for up to one year after patient discharge.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
February 10, 2009 - Sorin Group said today it acquired the Clearglide endoscopic vessel harvesting (EVH) product line of Datascope Inc., which enables less-invasive harvesting of vessels for use in coronary artery bypass grafting.
February 10, 2009 - W. L. Gore and Associates today said at the annual International Congress of Endovascular Interventions XXII, it received FDA approval to market a modified version of the GORE TAG Thoracic Endoprosthesis for the treatment of thoracic aortic aneurysms (TAAs).
February 10, 2009 - Hospitals will be eligible in Medicare’s 2009 fiscal year for up to an additional $53,000 in reimbursement when they implant a CardioWest temporary Total Artificial Heart (TAH-t) compared to subsequently transplanting a donor heart.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Codonics Horizon is three imagers in one – a dry film imager, color imager, and grayscale paper imager. Switching between film, color and paper requires no operator intervention. The all-in-one imager aims to provide high quality images while being affordable.
Integrity Disk Importer is designed to be an easy-to-use solution to read, reconcile and store medical studies from a disc into PACS, or allows DICOM images to be stored directly to Integrity from any network device.
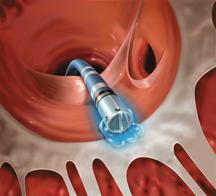
February 9, 2009 - Biosense Webster Inc. said Friday the FDA granted market clearance for the NaviStar ThermoCool Catheter for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation, when used with compatible 3D electroanatomic mapping systems.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
February 9, 2009 - Implantation of drug-eluting stents to treat degenerative in aortocoronary saphenous vein graft (SVG) lesions is safe, according to a study published in the Jan. 15 issue of the American Journal of Cardiology (Vol. 103, issue 2, Pages 199-202).
February 9, 2009 – Some patients who suffer a stroke as a result of a blockage in an artery in the brain may benefit from a clot-busting drug nine or more hours after the onset of symptoms, according to findings published in the online edition of Radiology.
February 9, 2009 - Emory University cardiologists are testing a modification of angioplasty known as postconditioning, designed to reduce permanent damage to the heart muscle after a heart attack.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 6, 2009 - Biosense Webster Inc., a Johnson & Johnson company, today said the FDA granted market approval to the NaviStar ThermoCool Catheter for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation, when used with compatible 3D electroanatomic mapping systems.
February 6, 2009 – The FDA has approved St. Jude Medical Inc.’s medical device system that allows a single defibrillation lead connection between a cardiac resynchronization therapy defibrillator (CRT-D) and the leads that send electrical impulses to the heart to treat the symptoms of heart failure.

February 6, 2009 - Medtronic Inc. today said it completed the acquisition of Ablation Frontiers Inc. for an initial payment of $225 million plus potential additional payments contingent upon achievement of certain clinical milestones.

 February 10, 2009
February 10, 2009