The Centers for Medicare and Medicaid Services (CMS) gathered public comments on its Stage 3 Meaningful Use (MU) requirements through May 30. The third and final set of MU rules are a core part of the plan to reform the American healthcare by leveraging health information technology (IT) to help reduce costs, eliminate redundancies, and convert from a fee-for-service to a fee-for-performance reimbursement system.
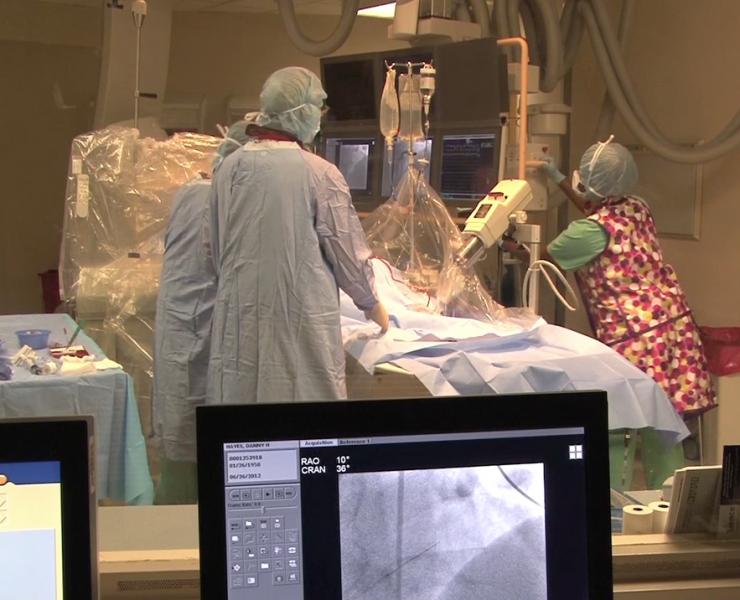
Cincinnati, Ohio-based The Christ Hospital Health Network’s Heart and Vascular Center is committed to delivering the highest quality of care to its patients. They recently deployed an integrated cardiovascular information system (CVIS) in order to support this commitment to quality.
NorthStar Medical Radioisotopes LLC has completed its first production-scale test run of the molybdenum-99 (Mo-99) aliquoting system installed at the University of Missouri Research Reactor (MURR) in Columbia, Missouri. The test and subsequent shipment of the resulting Mo-99 to NorthStar’s Madison facility is another milestone in the establishment of domestic production of the vital medical radioisotope.
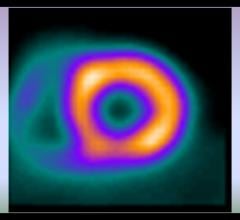
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
A new study shows that the recently developed Compact Light Source (CLS), a commercial X-ray source, enables computed tomography (CT) scans that reveal more detail than routine scans performed at hospitals today. With roots in research and development efforts at the Department of Energy’s SLAC National Accelerator Laboratory, the new technology could soon be used in preclinical studies and help researchers better understand cancer and other diseases.
The Organization for Occupational Safety in Interventional Fluoroscopy (ORSIF) announced the release of a new documentary film on the impact of chronic, low-level exposure to ionizing radiation on physicians that practice interventional medicine in fluoroscopy labs. The documentary features noted heart surgeon Edward Diethrich, M.D., founder of the Arizona Heart Foundation.
People with acute coronary syndrome (ACS) who undergo an angioplasty procedure and receive a heart stent are prescribed an oral antiplatelet (OAP) therapy and aspirin to help prevent a heart attack, a blood clot in their heart stent (stent thrombosis), or even death.[3]
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A retrospective review of 633 adults and children who underwent bioprosthetic pulmonary valve replacement (PVR) for congenital heart disease between 1996 and 2014 indicated that the risk of re-intervention was five times greater for children than adults. In addition, the likelihood of re-intervention decreased by 10 percent for each increasing year of age at surgery. Valve type was another important determinant of re-intervention, according to Rio S. Nomoto, BA, medical student at Tufts University School of Medicine, who presented the results of this research at the 95th American Association for Thoracic Surgery (AATS) Annual Meeting in Seattle on April 28.
The Spectranetics Corporation announced it is accelerating investments in the Stellarex drug-coated balloon angioplasty (DCB) platform for treatment of below the knee (BTK) peripheral artery disease. The company estimates this will represent a $150 million market opportunity by 2020.

Ultrasound can increase the rate at which heart cells beat, researchers from Drexel University report. In their paper “Ultrasound-Induced Modulation of Cardiac Rhythm in Neonatal Rat Ventricular Cardiomyocytes,” published ahead-of-print in the Journal of Applied Physiology, they describe the ultrasound settings that can change the beat frequency of cardiac cells.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
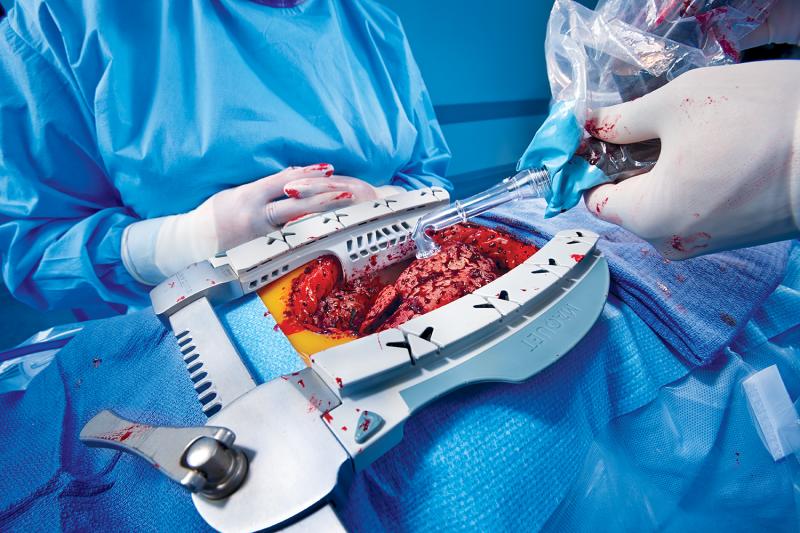
Transmyocardial laser revascularization (TMLR or TMR) was introduced in the late 1990s as a method to offer relief to patients with incapacitating angina that were poor candidates for other procedures such as coronary artery bypass graft surgery or balloon angioplasty and stenting. The basis for the procedure is use of a laser to bore small holes from the ventricle into the myocardium, allowing blood to flow directly into the heart muscle without the need to travel through blocked coronary arteries. The belief is that this process helps enable angiogenesis.
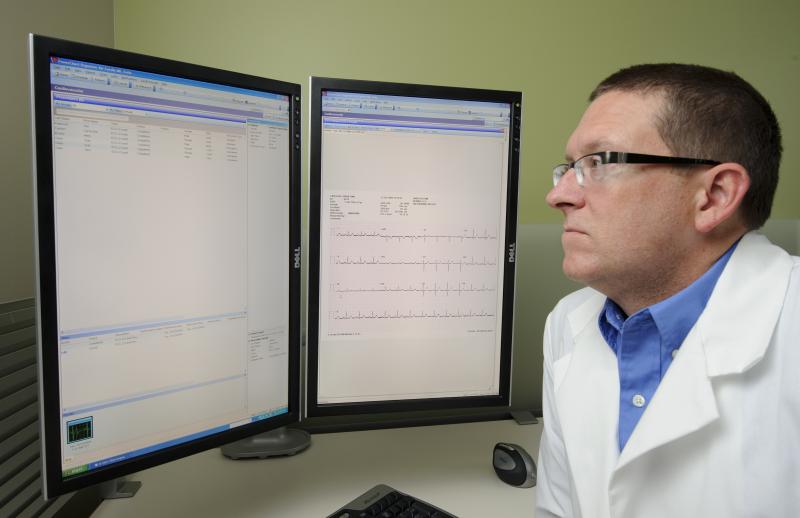
Hospitals with electronic health records (EHRs) for ischemic stroke patients did not demonstrate better quality of care or clinical outcomes for those patients when compared to similar hospitals without EHRs, according to a study published in the Journal of the American College of Cardiology.
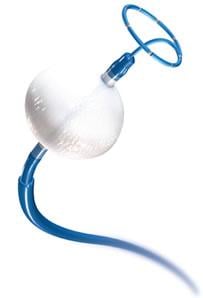
Medtronic plc announced the start of a clinical study to determine whether paroxysmal and persistent atrial fibrillation (AF) can be treated with a combination of two ablation procedures targeting different anatomical locations – specifically, the pulmonary veins and the renal arteries. Study patients will also receive an implantable cardiac monitor to track their heart rhythm on an automatic and continuous basis. AF is a cardiac rhythm disorder affecting an estimated 2.7 million people in the U.S.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
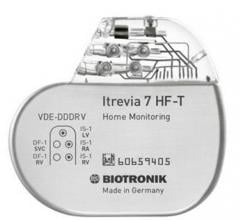
Biotronik announced that a unique physiologic sensor is now available in the latest family of cardiac resynchronization therapy defibrillator (CRT-D) devices. Closed Loop Stimulation (CLS) is the only cardiac sensor capable of appropriately adapting heart rate in response to physiologic demands independent of body movements or respiratory rate.
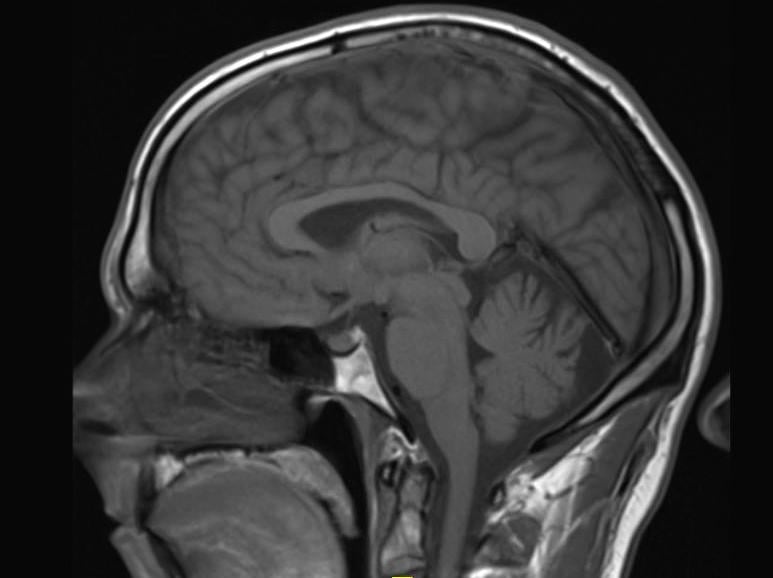
New research, published in the journal Radiology, suggests some types of a popular contrast agent used in magnetic resonance imaging (MRI) exams may remain in the brain for years, but that the long-term effects are unknown.
Carestream showcased the advanced interoperability of its Clinical Collaboration Platform (CCP) at the IHE-Europe (Integrating the Healthcare Enterprise) Connectathon held April 20-24 in Luxembourg. Carestream announced that the CCP product suite successfully passed all integration tests at the Connectathon including all profiles for its universal viewer, vendor-neutral archive (VNA) and image capture solutions. Carestream is also among the first VNA suppliers to implement HL7's new Fast Healthcare Interoperability Resources (FHIR) framework.

 May 07, 2015
May 07, 2015