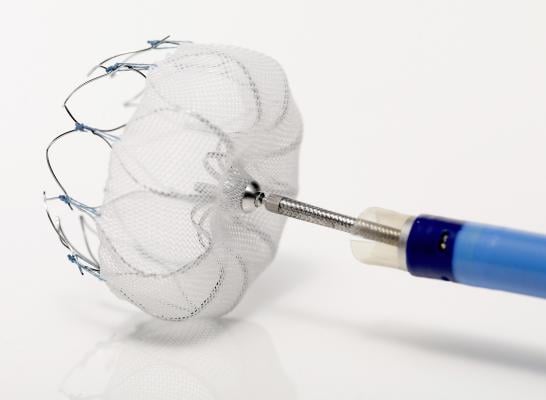
March 16, 2015 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval for the Watchman left atrial appendage closure device. The Watchman device offers a new stroke risk reduction option for high-risk patients with non-valvular atrial fibrillation who are seeking an alternative to long-term warfarin therapy. The device will be made available to U.S. centers involved in Boston Scientific clinical studies and additional, specialized centers as physicians are trained on the implant procedure.
The Watchman LAAC device is a catheter-delivered heart implant designed to close the left atrial appendage (LAA) in order to prevent the migration of blood clots from the LAA, and thus, reduce the incidence of stroke and systemic embolism for higher risk patients with non-valvular AF.
The device is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with non-valvular atrial fibrillation who are:
- At increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores;
- Are deemed by their physicians to be suitable for warfarin; and
- Have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared to warfarin.
"The Watchman device is an important step forward in stroke management for patients with AF," said Vivek Reddy, M.D., director of the Cardiac Arrhythmia Service at the Mount Sinai Medical Center and co-principal investigator of the PROTECT AF and PREVAIL studies. "We know that up to 40 percent of patients who are eligible for oral anticoagulation do not take it for numerous reasons, highlighting the need for additional treatment options. The Watchman device is a breakthrough treatment providing those patients who are suitable for warfarin with an implant-based alternative to long-term warfarin therapy while still reducing the risk of stroke."
The FDA approval of the device is based on the Watchman clinical program, which consists of numerous studies featuring more than 2,400 patients and nearly 6,000 patient-years of follow-up. The clinical program provided strong evidence that the Watchman device can be implanted safely and reduces the risk of stroke in eligible patients while enabling most patients to discontinue warfarin. Additionally, a meta-analysis of all of the randomized trial data demonstrated that while ischemic stroke reduction favored warfarin, the Watchman device provided patients with a comparable protection against all-cause stroke and statistically superior reductions in hemorrhagic stroke, disabling stroke, and cardiovascular death compared to warfarin over long-term follow-up.
For more information: www.bostonscientific.com


 August 28, 2023
August 28, 2023 









