Feds Helping Boost Technology in Rural America Via Telemedicine
Baxter Healthcare Corp. received FDA 510(k) clearance for its needleless V-Link Luer-activated device (LAD), an IV ...
Power Medical Interventions Inc. said the FDA cleared its intelligent iDrive surgical devices, which includes a suite of ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The SafeSeal Hemostasis Patch topical wound dressing is designed to decrease the time it takes to control bleeding from the puncture made into a blood vessel to perform an endovascular procedure.
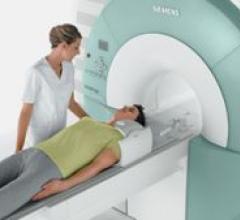
Siemens Medical Solutions received FDA 510(k) clearance for a 1.5T magnetic resonance imaging (MRI) system that is ...
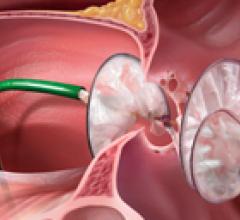
W. L. Gore & Associates (Gore) announced that the FDA granted approval for the use of GORE HELEX Septal Occluder with a modified catheter delivery system indicated for the transcatheter closure of atrial septal defect (ASD), providing a percutaneous ASD closure solution for very young patients.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

February 2008 - The traditional hospital cleaning methods of wiping down surfaces and mopping floors are now being ...
February 20, 2008 - Philips Medical Systems features at HIMSS 2008 enhancements to its speech recognition solution ...
Toshiba America Medical Systems Inc. recently introduced its Infinix VF-i/SP X-ray system, a universal cardiovascular ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The ACUSON X300 works to provide compact, portable color Doppler solutions for adult, pediatric, OR, EP and ...
February 20, 2008 - At HIMSS 2008, Siemens Healthcare will emphasize its image data management solutions for radiology ...
February 19, 2008 – A novel, fully bioabsorbable salicylate-based stent offers the potential to reduce adverse events associated with current drug-eluting stents (DES) and could be very beneficial to patients with coronary heart disease, according to a presentation at the Cardiovascular Revascularization Therapies 2008 (CRT) symposium.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
February 19, 2008 – Clinicians implanted the BioMatrix drug-eluting stent system into the first European patients in a live broadcast during the Joint Interventional Meeting (JIM), held in Rome, Italy, from February 13-15. The BioMatrix drug-eluting stent system, by Biosensors International Group Ltd., combines a biodegradable PLA and the company’s proprietary limus drug, Biolimus A9.
February 19, 2008 – The American College of Cardiology (ACC) urges the medical community to oppose cuts to medical ...
February 19, 2008 - Boston Scientific Corp. closed the sale of its Fluid Management and Venous Access businesses to ...

 February 20, 2008
February 20, 2008