Patients can have their cardiac issues remotely monitored from locations across the country with the use of portable devices and telephone or Internet connections through Raytel Cardiac Services.
Procedicus VIST is a simulator for catheter-based procedures providing relevant and realistic hands-on training for ...
The NICO2 Respiratory Profile Monitor is designed to monitor the patient side of the breathing circuit. NICO2 goes ...
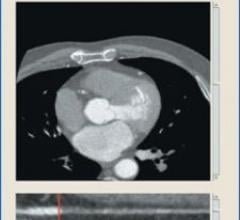
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Vanguard Dx Angiographic Catheter with patented RadiantFlow tip technology delivers contrast in a unique way ...
The Cardius 3 XPO triple-head camera reportedly features fourth generation High Definition Solid-State Detectors (HDSD) ...
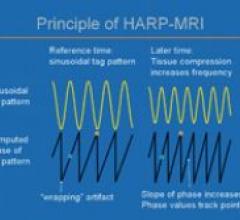
Diagnosoft HARP is software that assists physicians in the analysis of magnetic resonance (MR) images by providing ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The Pinnacle R/O II HiFlo Introducer Sheath is a 4-cm stiff introducer sheath that features a smooth transition that ...
The AtriCure Ablation and Sensing Unit (ASU) is a device that makes linear and transmural lesions in a matter of seconds ...
ScImage’s latest additions to its Web-based cardiology viewing suite, PicomEnterprise, reportedly allowing gated SPECT ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The COR Analyzer I by Rcadia Medical Imaging assists screening of triage patients for coronary artery disease. The ...
When used in conjunction with the ev3 embolic protection device, ev3’s PROTÉGÉ RX Carotid Stent has been FDA cleared for ...
March 19, 2007 — CardioNet, Inc., a provider of wireless mobile cardiac outpatient monitoring solutions, has announced ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 19, 2007 — Onset Medical Corp. has announced it has received FDA clearance to begin marketing the SoloPath ...
March 19, 2007 — AT&T Inc. has announced an agreement wherein it will upgrade the conference platform used by Medical ...
March 19, 2007 — Medtronic Inc. has launched its U.S. pivotal clinical trial for the Cardioblate Surgical Ablation ...


 March 20, 2007
March 20, 2007