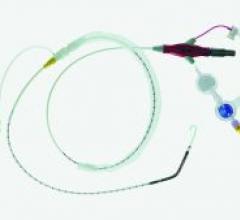
Safe delivery of the balloon or stent through fragile vasculature during percutaneous transluminal coronary angioplasty (PTCA) procedures is an important criterion for successful patient outcomes.

Cardiology fellows are expanding their knowledge in cardiovascular imaging. Not only do virtually all cardiology fellows get Level 2 training in echocardiography and many in nuclear cardiology but now, an increasing number of fellows are training in cardiac MR and cardiac CT.
July 10, 2008 - The Senate overcame partisan gridlock on July 9, 2008, and passed the Medicare Improvements for Patients ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
InTouch Technologies Inc. offers StrokeRESPOND to extend the functionality of its telemedicine, Remote Presence robotic ...
Thoratec Corp. received FDA approval of its PMA (premarket approval) application, allowing the use of its HeartMate II LVAS (left ventricular assist system) as a bridge-to-transplantation (BTT) in patients suffering from advanced-stage heart failure.
Northeast Monitoring’s DR200/HE is a combination 14-day Holter plus 30-day Event recorder integrated into a single unit ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
July 9, 2008 - New research shows catheter cryoablation is comparably effective to radiofrequency catheter ablation for the treatment of atrial flutter (AFL) and may have some safety advantages over the use of radiofrequency.
The FDA cleared GE Healthcare’s new LightSpeed CT750 HD, said to be the world’s first high-definition CT scanner that ...
Vascular Insights received 510(k) clearance from the FDA to market its ClariVein infusion catheter for infusion of physician-specified agents in the peripheral vasculature. ClariVein is a percutaneous, 2 2/3 Fr (0.035-inch) catheter, containing a rotating wire driven by a motor, that enhances fluid dispersion in the treatment area.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
July 10, 2008 - The XIV Congress of the Latin American Society of Interventional Cardiology (SOLACI) and the annual ...
The Abiomed Inc. Impella 2.5 cardiac assist device was FDA cleared in 2008 for use for partial circulatory support for periods up to six hours. The intra-aortic balloon pump (IABP) also has 510(k) clearance and approximately 110,000 are used each year in the U.S.
The FloTrac is reportedly the first minimally invasive hemodynamic monitoring device that connects to any existing arterial line and requires no manual calibration. The latest enhancement to the FloTrac software empowers clinicians with greater flexibility to trend and analyze the patient parameters in order to make better informed decisions, said the company.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
July 9, 2008 – The American Heart Association recently issued new guidelines for computed tomography (CT) and magnetic ...
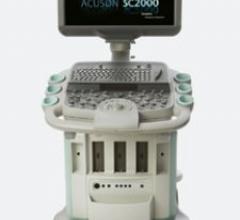
The ACUSON SC2000 volume imaging ultrasound system acquires nonstitched, real-time full-volume 3D images of the heart in ...
The Sorin Group said the FDA approved to market the CRT model of its OVATIO family, reportedly the smallest CRT defibrillator (CRT-D) available. The OVATIO CRT 6750 is shaped to offer ease of implantation and long-term patient comfort, and is designed to allow more flexibility in the management of cardiac resynchronization and antitachyarrhythmia therapy due to its Brady-Tachy Overlap (BTO).

 July 09, 2008
July 09, 2008