May 1, 2008 - Cardiothoracic surgeons will discuss the benefits of using new technologies to facilitate off-pump ...
May 30, 2008 - St. Jude Medical Inc. launched the TigerWire Steerable Guidewire, designed to enhance physicians' ability ...
May 1, 2008 - The clinical impact of the first-ever prohealing stent, OrbusNeich's Genous Bio-engineered R stent, is the ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
May 30, 2008 - St. Jude Medical Inc. launched the TigerWire Steerable Guidewire, designed to enhance physicians' ability to steer through challenging peripheral arteries, as the company extends its GuideRight portfolio of steerable guidewires.
May 1, 2008 – Royal Philips Electronics and BrainLAB said they would be collaborating on the integration of intra-operative angiography and image-guided surgery (IGS). The integration of angiography and IGS would provide surgeons with real-time information during interventions that may lead to more precise surgical procedures.
April 30, 2008 - Acusphere has submitted a New Drug Application (NDA) to the FDA for approval to market Imagify ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
April 30, 2008 - The PAS-Port Proximal Anastomosis System achieved its primary endpoint in a large, prospective ...
April 30, 2008 - A new study indicates that use of the minimally invasive W.L. GORE TAG Thoracic Endoprosthesis to treat descending thoracic aneurysms appears to be superior to open surgical repair in anatomically suitable patients.
April 30, 2008 - The surgeon working inside J.C. Bizzle's chest perched at an egg-shaped console a few yards from the ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 30, 2008 - Physio-Control Inc. has reached an agreement on a consent decree with the FDA regarding its quality ...
April 29, 2008 - Routing video and other vital information to and from the surgical team often requires installing ...
Medtronic's Defender embolic protection filter, available in Europe, is a device designed to get rid of emboli that can occur during a variety of stenting procedures.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 29, 2008 - HealthEast Care System publicized The National Brain Aneurysm Center and the www.brainaneurysmcenter.org Web site, formerly the HealthEast Neurovascular Institute, focusing on complex neurovascular conditions such as aneurysm, stroke, vascular malformations, brain tumors and more.
April 29, 2008 - The FDA has approved the LipiScan Coronary Imaging System, an angiography laser scanner by InfraReDx Inc., which is intended to characterize fatty deposits in coronary vessel walls using near infrared diffuse spectroscopy.
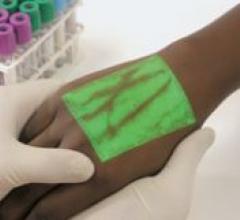
The VeinViewer by Luminetx is a vascular imaging system that allows physicians, nurses and other healthcare ...

 April 30, 2008
April 30, 2008