Siemens Medical Solutions previewed a hand-held ultrasound unit, the Acuson P10, intended primarily for triage and screening applications in acute care settings. In the future, personal imaging tools such as this could improve the standard of care for inpatient monitoring, critical care, outpatient specialty visits and bedside exams.
ScImage announced that it is delivering a fully customizable reporting and workflow documentation solution for vascular procedures. The reporting solution will be offered as an add-on to the company’s award winning Enterprise PACS solution, PicomEnterprise.
The results of Boston Scientific's pivotal SPIRIT III clinical trial reaffirmed prior safety and efficacy data for the market-leading TAXUS Express2 Paclitaxel-Eluting Coronary Stent System and provided early positive data for the XIENCE V (PROMUS) Everolimus-Eluting Coronary Stent System.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
ZOLL Medical Corp. has received FDA clearance to market and sell the ZOLL M Series with Real CPR Help technology. The device enables rescuers to instantly see and hear how well they are performing the rate and depth of CPR chest compressions. The M Series is the Company’s best-selling product to date. The approval extends Real CPR Help to ZOLL’s complete defibrillator product line.
Boston Scientific Corp. announced two-year results from the TAXUS V In-Stent Restenosis (ISR) trial, demonstrating that the TAXUS Express2 paclitaxel-eluting coronary stent system met its primary endpoint in the treatment of in-stent restenosis - the regrowth of diseased tissue into a previously stented artery utilizing bare-metal stents - compared to vascular brachytherapy.
St. Jude Medical Inc. says it has received FDA approval of a new cardiac rhythm management device designed to help physicians manage heart failure (HF) patients, including patients who have or may develop atrial fibrillation (AF).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
A new MRI monitor to complement the Maglife product line, the Maglife light makes MRI monitoring within reach of virtually every MRI Center. It can handle all monitoring needs and is compatible with MRI scanners up to 3T.
When used in conjunction with the ev3 embolic protection device, ev3’s PROTÉGÉ RX Carotid Stent has been FDA cleared for the treatment of carotid artery disease in patients who are at high-risk for adverse events from carotid artery surgery. The cleatance was supported by the CREATE (Carotid Revascularization with ev3 Inc.
VIASYS Healthcare is showcasing the launch of the MasterScreen CPX, a product that combines 40 years of CPET experience into a compact unit.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Pfizer says the FDA has approved the company’s Lipitor (atorvastatin calcium) Tablets to reduce the risk of nonfatal heart attacks, fatal and non-fatal strokes, certain types of heart surgery, hospitalization for heart failure, and chest pain in patients with heart disease — making Lipitor the first cholesterol-lowering medication to receive FDA approval for the reduction of the risk of hospit
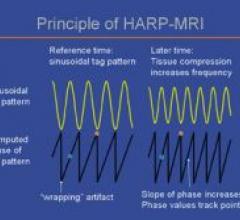
Diagnosoft HARP is software that assists physicians in the analysis of magnetic resonance (MR) images by providing quantitative measurements and visualization of regional heart function. Based on technology from Johns Hopkins University, it’s the first FDA-cleared software designed for the analysis of tagged MR images.
The NICO2 Respiratory Profile Monitor is designed to monitor the patient side of the breathing circuit. NICO2 goes beyond conventional capnography to measure breath-by-breath volumetric CO2 and takes the guesswork out of ventilation management from setup to weaning.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Coronary computed tomography angiography (CCTA) has recently made significant technological and clinical advances. Submillimeter slice thickness, increased detectors and reduced acquisition times have multiplied the number of patients that can benefit from noninvasive diagnostic imaging.
Rcadia Medical Imaging Ltd., an Israel-based developer of novel computer-aided diagnostic software, has received FDA clearance to market its COR Analyzer I, which assists screening of triage patients for coronary artery disease.
Exercise Tolerance Testing (ETT) is a diagnostic tool consisting of the RHE cycle, that is used in conjunction with scientifically validated exercise testing protocols to create a more accurate, sensitive and comfortable test, according to the company.

 March 27, 2007
March 27, 2007