BioTelemetry Inc., a wireless medical technology company focused on the delivery of health information, announced that recently published research from an observational study of more than 200,000 patients demonstrates significant in-hospital cost savings in the 12 months following diagnosis using mobile cardiac telemetry (MCT).
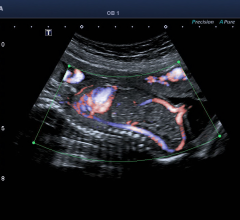
Ultrasound will be able to depict blood flow with more detail and accuracy using Toshiba America Medical Systems Inc.’s work-in-progress ultrasound capability, which is the latest advancement to Toshiba’s Aplio 500 ultrasound system.
Double Black Imaging demonstrated its full line of auto-calibrating LED displays at the Radiological Society of North America Annual Meeting (RSNA 2013).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

Philips Healthcare introduced the IQon Spectral computed tomography (CT) system at RSNA 2013. It is the world’s first spectral-detector CT system built from the ground up for spectral imaging. It uses color to identify the composition of an image without involving time-consuming protocols.
At RSNA 2013, GE Healthcare introduced the Discovery Interventional Guidance System (IGS) 740, a 510(k)-pending mobile X-ray angiography system with a 41 x 41 cm detector. The system is aimed at interventional radiology market.
Philips unveiled the Vereos PET/CT fully digital positron emission tomography/computed tomography (PET/CT) imaging system at the Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
BioCardia Inc., a cardiovascular regenerative medicine company, announced positive 12-month results for the randomized Transendocardial Autologous Cells (MSC or BMC) in Ischemic Heart Failure Trial (TAC-HFT).
Thomas Jefferson University researchers discovered that the formation of blood clots follows a different molecular route in African Americans versus European Americans, providing a new understanding of the effects of race on heart disease.
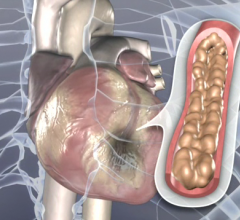
TherOx Inc. released results from the company's multicenter Investigational Device Exemption (IDE) pilot study that showed a 9.6 percent infarct size at 30 days in high-risk patients treated with its next-generation system for Supersaturated Oxygen (SSO2) Therapy. This therapy is intended to provide interventional cardiologists with the first treatment option beyond percutaneous coronary intervention (PCI) to salvage heart muscle in heart attack patients. The study results were presented at the 25th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium, sponsored by the Cardiovascular Research Foundation.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
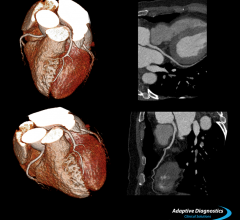
Toshiba introduced its Adaptive Diagnostics computed tomography (CT) technology, which simplifies complex exams, lowers dose and improves diagnostic accuracy and reproducibility, at the Radiological Society of North America Annual Meeting (RSNA 2013).
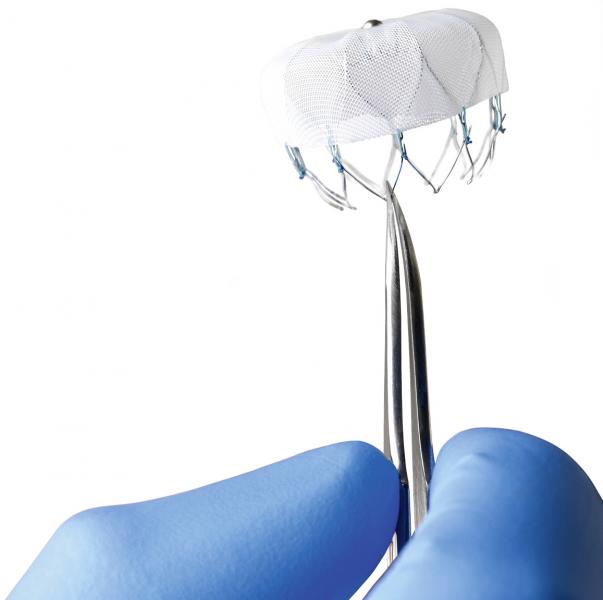
The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee voted favorably Dec. 11 in a 13-1 vote that the benefits of the Watchman Left Atrial Appendage Closure (LAA) device outweigh the risks. The company expects a decision from the FDA in the first half of 2014.
Novarad’s NovaCardio electrocardiogram (ECG) integrates with cardiology picture archiving and communication system (PACS)/cardiovascular information system (CVIS) to enable digital ECG reading, annotation, manipulation, storage and easy reporting. NovaCardio ECG was debuted at the Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The National Cheng Kung University Heart Science and Medical Devices Research Center (NCKU HSDMRC) and the Division of Cardiothoracic Surgery, Department of Surgery, Duke University, Durham, NC, USA, jointly carried out a cardiac assist device implantation on a calf in October.
AccessClosure Inc. announced that the U.S. Court of Appeals for the Federal Circuit denied a request by St. Jude Medical Inc. to rehear an appeal on a double patenting ruling concerning its U.S. Patent No. 7,008,439 (the “Janzen ’439 patent"), covering technologies to close holes in arteries.

For patients with stable coronary artery disease (CAD) who are not experiencing a heart attack and an abnormal stress test, treatment of their narrowed arteries by the common procedure of angioplasty may not provide additional benefits compared to drug therapy alone.

 December 16, 2013
December 16, 2013