September 9, 2009 – Royal Philips Electronics unveiled its PageWriter TC50 cardiograph, which uses gender-differentiated criteria to assist in the diagnosis of heart disease in women, where symptoms may be different from men. The PageWriter TC50 was launched at the European Society of Cardiology (ESC) Congress 2009 Aug. 28.
September 9, 2009 - TeraMedica Healthcare Technology, a Milwaukee-based medical informatics company, announced the USPTO granted it the U.S. patent number 7,583,861 for its Evercore Smartstore solution, a clinical data management technology.
September 9, 2009 – ProSolv CardioVascular enhanced its outcome reporting capabilities by partnering with Cedaron Medical Inc., a developer of quality measurement solutions, to provide the complete registry support that facilities need for ACC, national, and state clinical performance reporting.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
September 9, 2009 – Abiomed Inc. said today its percutaneous Impella ventricular assist device will be highlighted in several clinical sessions at the upcoming TCT 2009 scientific meeting Sept. 21-25, in San Francisco.
September 9, 2009 – CardiacAssist Inc. said yesterday it will exhibit its TandemHeart System in San Francisco at the TCT 2009 and will highlight speakers who have used the device in their cath labs.
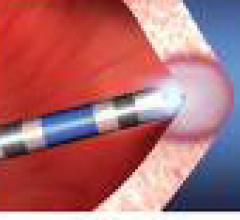
September 8, 2009 – Endosense, a medical technology company focused on improving the efficacy, safety and reproducibility of catheter ablation for the treatment of cardiac arrhythmias, said last week it received $36 million in financing to fund the European commercialization and U.S. premarket approval (PMA) clinical study of the company’s TactiCath force-sensing ablation catheter.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September 8, 2009 – Actelion Pharmaceuticals U.S. Inc. recently announced the first commercial sales of a new 20 microgram per milliliter (mcg/mL) formulation of Ventavis, for the treatment of New York Heart Association Class III and IV pulmonary arterial hypertension (PAH).
September 8, 2009 – Siemens Healthcare and SurgiVision Inc. today announced an agreement for the codevelopment and commercialization of a real-time magnetic resonance image (MRI)-guided cardiac electrophysiology (EP) system.
The HI-TORQUE Balance Middle Weight (BMW) Universal II Guide Wire offers the same performance of Abbott’s HT BMW UNIVERSAL wire, but includes the two advanced coating technologies and better coating durability for longer procedures
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The Perclose ProGlide 6 Fr. Suture-Mediated Closure System delivers a secure, reliable vessel closure to provide complete tissue apposition resulting in primary healing, reduces time to hemostasis, ambulation and discharge, and does not have any reaccess restrictions.
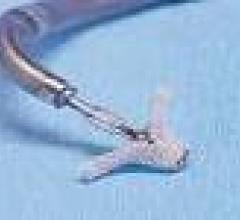
September 3, 2009 – Percutaneous cardiac valve maker Evalve Inc. announced treatment this week of the first group of patients with the MitraClip system at the Karolinska University Hospital in Solna, Stockholm, Sweden.
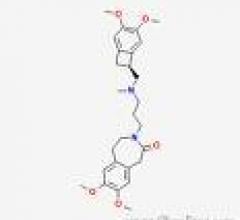
September 3, 2009 – Important new findings in patients with angina participating in the BEAUTIFUL study show that there is a 42 percent reduction in heart attack with Procoralan (ivabradine), as presented today at the European Society of Cardiology (ESC) Congress.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
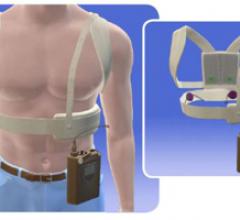
September 3, 2009 – ZOLL Medical Corp. received approval from the FDA to market and sell a new model of the LifeVest wearable defibrillator.
The HI-TORQUE Versacore .035 Guide Wire is a peripheral guide wire is designed for routine diagnostic and device delivery for balloons and stents in peripheral vessels. Versacore features a soft, shapeable tip to provide safe access to peripheral lesions and enhanced visibility under fluoroscopy.
September 3, 2009 – On Tuesday, Aug. 11, an 82-year old New Orleans resident with severe aortic stenosis successfully had a heart valve replaced at Ochsner Medical Center using the same technique as angioplasty, a far cry from the traditional open heart procedure.

 September 09, 2009
September 09, 2009