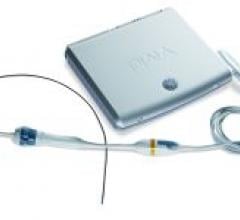
FlowCardia Inc., a medical device company developing a portfolio of endovascular devices for the treatment of coronary and peripheral chronic total occlusions (CTOs), highlighted its CROSSER 14P, CROSSER 14S and CROSSER 18 CTO Recanalization Catheters at TCT 2008.
The Cordis Aquatrack Hydrophilic Guide Wire is designed to facilitate access to the most tortuous anatomy.
October 20, 2008 – At TCT last week Abbott announced two-year data from 30 patients in its ABSORB clinical trial, demonstrating its bioabsorbable drug eluting stent successfully treated coronary artery disease and was absorbed into the walls of treated arteries within two years, leaving behind blood vessels that appeared to move and function similar to unstented arteries.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
At TCT 2008 AGA Medical Corp. highlighted its self-expanding AMPLATZER Muscular VSD Occluder for complex ventricular septal defect (VSD) repair in patients considered to be high-risk for standard transatrial or transarterial surgical repair.
October 16, 2008 - Lumen Biomedical Inc. said that Principal Investigator Subbarao V. Myla, M.D., presented the results ...
October 17, 2008 - Boston Scientific Corp. announced results at TCT from an analysis of the HORIZONS-AMI trial showing a ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
October 17, 2008 - Berlin Heart Inc. said yesterday that its EXCOR Pediatric ventricular assist device (VAD) has ...
October 17, 2008 - Elixir Medical Corp. today announced results from three multicenter first-in-man studies of Novolimus ...
October 17, 2008 - The Medicines Company’s Angiomax (bivalirudin) significantly reduced cardiac-related death, improved ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The GE Innova cardiovascular X-ray system will soon be aided by the new Innova Dose, Innova 3D and Innova with Stent Technologies features. GE’s Innova Dose package aims to deliver superb image quality and custom dose management.
October 16, 2008 - At TCT 2008, Volcano Corp. launched its new PrimeWire Pressure Guide Wire, and the s5-FFR Option for its existing installed base of s5 and s5i imaging consoles in the U.S.
The FDA has given GE Healthcare clearance to market the Vivid i with Intracardiac echo (ICE), a system designed to provide high performance ultrasound in a space saving design.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
October 16, 2008 - The one-year follow-up of the FAME study (FFR vs. angiography for multivessel evaluation) was presented at TCT 2008 and confirms the beneficial clinical and economic impact of FFR when used routinely to guide treatments of multi-vessel disease.
October 15, 2008 - Boston Scientific Corp. announced estimated U.S. market shares for September for its two drug-eluting ...
October 15, 2008 – Neovasc Inc., a new specialty vascular device company comprised of the former Medical Ventures Corp ...

 October 19, 2008
October 19, 2008