
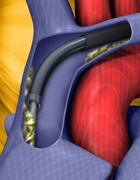
The Spectranetics Laser Sheath comes in various sizes to accommodate different size leads.
Cardiac rhythm device reliability and the approach to managing complications associated with these devices have recently been the topic of numerous medical and nonmedical publications. As the number of implanted cardiac rhythm devices continues to grow and the devices are implanted in patients with longer life expectancies, the need for lead management and lead extraction is expected to grow as well. It is now recognized that transvenous lead extraction is a central treatment in patients with cardiac rhythm devices and pathologic lead conditions.
Proper lead management requires an understanding of lead design and the pathophysiology of mechanical and clinical issues associated with lead dysfunction. The safety and efficacy of transvenous lead extraction varies widely and the number of trained physicians lags behind the current demand for this procedure. In May 2009 the Heart Rhythm Society (HRS) updated its expert consensus recommendations for transvenous lead extraction.
Watch the 2017 VIDEO "EP Lead Extraction Strategies," an interview with Bruce Wilkoff, M.D., director of cardiac pacing and tachyarrhythmia devices at Cleveland Clinic.
Indications for Lead Extraction
The indications for lead extraction can be divided into several categories: infection, malfunction, upgrade (abandoned/ unused lead), venous occlusion/stenosis and safety alerts.
Infection
The rate of device infection is growing at a greater rate than device implantation. Data suggests that the rate of device infection at the time of replacement and/or upgrade approaches 5-7 percent. Medicare data from 1990-1999 identified a 124 percent increase in the rate of cardiac device infection. Other data from a national hospital discharge survey identified a 310 percent increase in hospitalizations for cardiac device infections with the most dramatic increase being for implantable cardioverter defibrillator (ICD) infections. This was associated with a greater than two-fold increase in the risk of in-hospital death.
It is no longer acceptable to treat device infections in a “conservative” manner with antibiotics alone. Curative therapy nearly always requires removal of the entire pacemaker or defibrillator system from the infected individual, with re-implantation of a new system at an alternate site. Even for an isolated infection of the device pocket, complete system removal is indicated. This has been addressed in the most recent consensus guidelines where isolated pocket infection is now designated as a class I indication for complete system removal.
Venous Occlusion
As patients are being implanted with cardiac rhythm devices at younger ages and life expectancies continue to increase, a greater number of patients are being seen requiring system revision and upgrade. Increasingly patients are being seen with abandoned or unused leads and venous occlusions are becoming more widely recognized. The presence of venous obstruction is likely to become a common issue challenging all device-implanting physicians. While the rate of symptomatic occlusion is low, estimated at 0.35-3.5 percent, the rate of asymptomatic occlusion may be a high as 50 percent.
There are a variety of methods and tools to deal with venous occlusion in the patient requiring system revision or upgrade. The existing system can be abandoned with a new system on the contralateral side. A contralateral lead can be placed and tunneled across the chest to the existing system; or an epicardial lead can be placed via thoracotomy and tunneled to the existing system. Each of these approaches has significant limitations and drawbacks. One of the most effective ways of obtaining venous access in the patient with a venous occlusion is lead extraction, even of functional leads. Through the use of the extraction sheath, a lead can be removed and venous access obtained. This is true not just for veins such as the subclavian and innominate, but also for more central structures such as occlusion in or near the superior vena cava. Numerous reports in the literature have demonstrated the utility of lead extraction to facilitate device upgrade in the patient with venous occlusion.
Abandoned, Unused and Nonfunctional
Management of patients with abandoned, unused, nonfunctional leads or leads with safety alerts has been a topic of increasing attention over the last several years. Proper management of patients with these leads is critical. The failure rate of pacemaker and defibrillator leads varies widely depending on the age and model of the lead. Historically, the perceived risk of extraction has limited the referral and performance of this procedure to patients with life-threatening situations, but experience and the development of newer tools have influenced the outcomes of transvenous lead extraction. Particular attention should be paid to the type of lead that is under advisory with assessment of the risks versus benefits of lead extraction in the individual patient.
The recent, four-year retrospective LExICon (Lead Extraction in Contemporary Settings) study of 1,449 consecutive patients undergoing excimer laser assisted lead extraction has shed light on the current safety and efficacy of laser-assisted lead extraction. In the study, 96.5 percent of the 2,405 leads attempted were completely extracted and clinical success was achieved in 97.7 percent of patients.
Importantly, major adverse events directly related to lead extraction occurred in 1.4 percent of patients.
Operator experience is important in determining clinical outcome. In the LExICon study, extraction at a less experienced center (less than or equal to 60 cases) was associated with procedural and clinical failure. These findings were in agreement with other published data demonstrating a significant learning curve for this procedure. Therefore, physicians should consider their extraction experience when deciding to perform this procedure and consider whether extractions should be referred to higher volume centers.
New Approaches to EP Lead Management — Lead extraction strategies offered by EP Expert Bruce Wilkoff
Watch a VIDEO demonstrating how to use the Bridge Occlusion Balloon.
Editor’s Note: Avi Fischer is the director of pacemaker and defibrillator therapy and cardiac arrhythmia service at Mount Sinai Medical Center in New York, N.Y.


 January 29, 2026
January 29, 2026 









