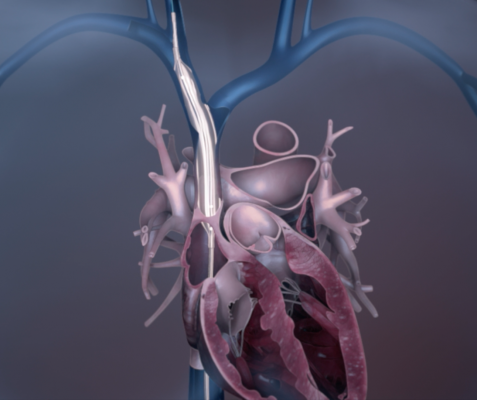
An illustration of how the Bridge balloon can seal the SVC after an accidental tear from lead extration.
May 17, 2017 — A new intravenous occlusion balloon designed to seal any accidental tears in the superior vena cava (SVC) during lead extraction procedures was shown it can provide a bridge to surgical repair and help reduce the usual 50 percent mortality associated with the complication. In the year since its introduction, the Spectranetics Bridge is credited for assisting in saving numerous lives, according to data presented at Heart Rhythm 2017, Heart Rhythm Society’s 38th Annual Scientific Sessions.
Central venous vascular tears constitute the potentially most lethal complication encountered during cardiac-implantable electronic device lead extraction. Historically, the case fatality rate of these events has been 50% due to blood loss prior to getting the patient into an operating room and opening them up for surgical repair.
Watch a VIDEO demonstrating how to use the Bridge Occlusion Balloon.
This study reviewed data from the MAUDE database. an FDA-maintained mandatory (hospital and manufacturer) registry of adverse events related to medical devices. All reports from July 1 - Dec. 31, 2016 involving Spectranetics extraction tools were reviewed independently by two physicians to identify instances of superior vena cava tears. Those cases were then analyzed for patient demographics, repair strategies and index hospital mortality.
The study included 68 adverse events reported from lead extractions involving Spectranetics tools. From those reports, 35 instances of superior vena cava tear were identified. In seven cases, the occlusion balloon was deployed after a tear was suspected and before performing open repair. The remaining 28 cases were managed with open surgery, but without endovascular occlusion. In all seven cases (100 percent) involving balloon use, the patient survived the index hospitalization. Without the aid of the balloon, 13 (46.4 percent) survived to discharge (p=0.0125).
Among the patients whose treatment included the endovascular balloon, five (71.4 percent) were female, median age was 60 years, and median lead age was 13 years. Four of the patients were treated without need for cardiopulmonary bypass, and there were no signs of neurological deficits prior to discharge. From July 2016 through December 2016, patients with superior vena cava tears were more likely to survive when treatment included an endovascular occlusion balloon, researches found.
Roger G. Carrillo, M.D., chief of surgical electrophysiology, University of Miami Hospital, Miami, Fla., who presented the data at HRS, said the balloon deploys in under two minutes, occluding an SVC tear and stemming blood loss by 90 percent, on average in 90 percent of patients.[1,2,3] When deployed, the Bridge Occlusion Balloon provides acceptable hemostasis for at least 30 minutes.[4] The device is intended to provide temporary occlusion while the patient is transitioned to surgical repair, providing a “bridge” to surgical repair.
“Bridge is a path to better patient outcomes in lead extraction. The compliance of the balloon makes the difference. I now consider the device an essential part of the clinical workflow,” Carrillo said.
EP lead management expert Bruce Wilkoff, M.D., Cleveland Clinic, said the device is one of the most important new developments in lead extraction technology. He said the balloon offers a safety net to minimize the effect of a potentially catastrophic SVC tear. While rare, he said this complication has made some physician shy away from referring patients for lead extractions in the past.
“Bridge is now part of our standard lead extraction protocol,” Wilkoff said.
About Lead Management
There are more than 7 million pacemakers and implantable cardiac defibrillators (ICDs) implanted worldwide, 700,000 new devices implanted each year and 1.5 million new leads implanted annually. These devices and the leads that connect them to the heart need ongoing management and maintenance. Sometimes this includes removal due to lead damage, lead or device infection, or manufacturer advisory. Laser lead extraction has been a treatment option for more than 20 years. Multiple studies show a procedural success rate of more than 97% demonstrating safety, effectiveness and efficiency of laser lead removal. In 2014, Spectranetics introduced mechanical tools into its comprehensive portfolio of lead management solutions. In 2016, the Bridge Occlusion Balloon launched to address the rare event of a tear in the superior vena cava (SVC).
New Approaches to EP Lead Management — Lead extraction strategies offered by EP Expert Bruce Wilkoff
Read the article “Advances in Transvenous Lead Extraction.”
Read the article “Strategies, New Technologies Aid Lead Management.”
Watch the VIDEO "EP Lead Extraction Strategies," an interview with Bruce Wilkoff, M.D., director of cardiac pacing and tachyarrhythmia devices at Cleveland Clinic.
For more information: www.spectranetics.com
References:
1. Document on file D027562. Bridge can be fully deployed in under one minute (53 seconds) in an animal model when pre-positioned on a guidewire, or in under two minutes (1 minute, 46 seconds) when not pre-positioned.
2. Document on file D027561. When deployed, the Bridge occlusion balloon reduces blood loss by up to 90%, on average, in an animal model of an SVC tear. Testing was conducted in a heparinzed porcine model which has shorter SVC length than is typical in humans. A balloon design scaled for use specifically in the porcine model was used in generating this data.
3. Document on file D027563. The balloon will cover the length and diameter of the SVC in 90% of the population as determined by analysis of 52 patients (N=52, % Male=48.1, Average Age 47.1 ± 16.5, Age Range 63 (18 to 81 years), Average Height 170.8cm ± 10.6, Height Range 40.6cm (152.4 to 193cm), Average BMI 29.8 ± 7.2, BMI Range 32.1 (18.2 to 50.3)).
4. Document on file, D026197. In an animal model with SVC tears up to 3.5 cm, with 2 pacing leads and 1 ICD lead.
4. Carrillo. “The LBCT: Compliant Endovascular Balloon Reduces the Lethality of Superior Vena Cava Tears During Transvenous Lead Extractions.” Presentation at Heart Rhythm 2017, May 12, 2017.



 January 05, 2026
January 05, 2026 









