As a cardiovascular technology magazine editor, I keep tabs on what technology is in development and often have the opportunity to see the most cutting edge technology up close prior to U.S. Food and Drug Administration (FDA) approval. While I am knowledgeable about healthcare and the clinical aspects of cardiology, I am a journalist, not a clinician. This gives me a different prospective on technology innovations and how I evaluate them. However, when comparing notes with clinical end-users, I discovered there are certain universal truths to consider when reviewing new technology, be it a new device, procedure or IT system:
The KISS Principle
The “keep it stupid simple” (KISS) principle is a time-honored axiom that should be applied to all new technology. Regardless of how complex its design or programming, if most people do not find it simple to use, the technology will have difficulty seeing wide-spread use. Also, the more complex something is to use and the more steps involved, the more prone to error it becomes.
Just because the main operators of a device in the cath lab will be physicians does not mean it should not be made idiot proof. A good example of this is with vascular closure devices, most of which require several steps to take place in a specific order for the device to work properly. A few years ago, Abbott Vascular found physicians could not remember the order of steps for its vascular closure device. The vendor improved the device using the KISS principle by simply adding numbers to each of the buttons on the device handle so the proper order could be followed more easily.
I have found the more complex the technology, the more it seems to be limited to use mainly by university teaching hospitals for research, rather than as an everyday tool. An example that comes to mind was the first generation Infraredx LipiScan Coronary Imaging System, which uses near infrared diffuse spectroscopy to characterize the chemical makeup of plaque in coronary vessels as blobs of colors on a screen. When I first saw the system I remember thinking although it was a cool idea, no one was going to use this technology because it was too difficult to figure out where in the vessel the plaques were located because all you saw was a chemogram without reference to the anatomy. When Infraredx combined its technology with intravascular ultrasound (IVUS) to show a fused image of both the vessel anatomy and morphology, the systems popularity among cardiologists changed overnight because it became easier and more practical to use.
Work Flow Efficiency
If a technology or software program requires 20 steps to complete an action, reducing the number of steps will save time, boost efficiency and increase utilization. This plays a big role in software, because if filing out a patient procedure or imaging report requires numerous steps and is time consuming, the physicians and staff using it will find work-arounds to cut corners, procrastinate using the system and complain. If the software is streamlined to reduce the number of mouse clicks in half or more from an older system for the same task, and incorporates automation (use of drop-down menus of terms, devices and drugs; common paragraph descriptions; automatic importation of data fields from hemodynamic systems; automated completion of NCDR data submission forms; etc.) the system will be much better received. In addition, even the reduction of a few steps or mouse clicks can save a few seconds or minutes per patient, which will translate into hours, days or weeks of saved time over the course of a year.
Technology Needs to be Intuitive
Also incorporating the idea of KISS is the need for technology to be intuitive. If a new picture archive and communications system (PACS), cardiovascular information system (CVIS), imaging system or cath lab device requires a couple weeks of on onsite training and trouble shooting when it goes live, it should be a red flag that the system is not intuitive to use. Most technology users want plug and play ability and would like to be able to use a new technology quickly with a steep learning curve.
Take for example smartphones. Few people actually read their owner’s manuals for their iPhone, because Apple realized the key to effective user interfaces is the ability for anyone to be able to pick up the device and start using it intuitively. This idea of intuitive design is now incorporated into many medical devices. Today, it is not enough for a device to function after someone goes through several days of training and reads through a 200-page manual, it needs to be a no brainer on how to operate it and users need to be able to do so quickly.
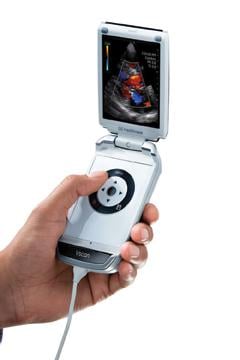
An example of this was Siemens Healthcare’s introduction of the Acuson P10 pocket ultrasound system in 2007. Siemens reduced the size of a standard ultrasound, but left all the complicated-looking knobs and switches that only an experienced ultrasound tech knows how to use, so it was not very popular. GE Healthcare also developed a hand-held ultrasound system with the same level of complexity, but held off introducing it. Instead, they redesigned the whole system to use an iPod type control system so it could be operated with one hand so the other hand was free to manipulate the transducer. The simplicity of the GE Vscan system meant any high school student has the intuitive technical ability to pick up this device and begin using it in a short time. GE has been selling Vscans like hot cakes since it was introduced in 2010 because any point-of-care physician can use the device without the need for extensive ultrasound training.
Economics of a New Technology
A big fly in the ointment for the widespread adoption of many new technologies is cost. In today’s world, there needs to be a clear, quantifiable return of investment (ROI). This is especially true for new medical devices that are competing with a long-established standard of care, where the new technology must show either a cost-benefit over the older technology, or must show a big improvement in patient outcomes to justify the added expense.
When conducting this type of evaluation, revolutionary new technologies that fundamentally change patient outcomes for the better are no brainers, even if it is more expensive. This was certainly true with the introduction of transcatheter aortic valve replacement (TAVR) devices for extreme-risk surgical patients verses the old standard of medical therapy alone. The Edwards Sapien TAVR valve was shown to be so effective at keeping these very sick patients alive compared to the standard of care using drugs alone, that the safety monitoring board abruptly ended the medication only arm of the study. The survival benefit and safety was very clear for the Sapien valve, and patients in medication arm were all transferred to the TAVR arm.
However, if a device shows only an incremental increase in benefit, say a 1-2 percent or less improvement over existing devices, the question may become one of economics rather than device effectiveness. This is what happened with drug-eluting stents in the last decade, where key vendors where in an arms race to build a better stent and capture a large share of the then lucrative coronary stent market. This included extremely expensive, blockbuster clinical trials, massive R&D efforts and lawsuits, and counter suites to try to slow down the competition. However, this came to a halt with the SPIRIT IV trial results in 2010, which proved the Abbott Xience stent was the best in the market for patient outcomes and beat out the long-time market leader, the Boston Scientific Taxus stent.
However, the victory only just edged out Taxus. Stent thrombosis improvements were measured in fractions of less than 1 percent, and ischemia-driven target-lesion revascularization improvements were less than a 2.6 percentage-point absolute reduction. This proof that Abbott was the top performer by only a small margin came at the cost of nearly a billion dollars. Few vendors are willing to shell out that sort of cash today to conduct similar sized trials that will be powered with enough patients to show only small incremental improvements.
In reality, all the drug-eluting stents on the U.S. market today are good devices and all have similar patient outcomes. This has led to stents becoming commodity items, where lower pricing can often trump slightly better trial data.
While cost is a major factor, hospitals should consider if a more expensive technology may help save money in the long run for specific patient populations. The intra-aortic balloon pump (IABP) has been the gold standard of care for hemodynamic support since the 1970s. The 40-year-old technology has been challenged in the past decade by two percutaneous ventricular assist device (pVAD) vendors, Tandemheart and Abiomed. While the cost of pVADs has prohibited their widespread use, they have found a niche among their supporters for very high-risk patients that require more hemodynamic support than IABPs can offer. Advocates point to studies where pVADS actually helped save money over the long-term, because the patients recovered more quickly.




 November 14, 2025
November 14, 2025 









