In Dr. Renu Virmani's rabbitt iliac models, next-gen stents Endeavor and Xience showed promising re-endothelialization at 28 days.
Examples of physicians’ declining use of drug-eluting stents are growing.
According to Dr. Louis Cannon, Cardiac & Vascular Research Center of Northern Michigan, the frequency at which drug-eluting stents are being used to treat patients with coronary artery disease (CAD) has decreased to about 70 percent at his facility. The New York Times predicted in October 2006 that DES usage could decrease from more than 90 percent usage down to 80 percent. Martin Leon, M.D., Cardiovascular Research Foundation, during a roundtable discussion at the 2007 DES Summit in New Orleans, estimated that the frequency of DES implantation had fallen by 20 percent in the last six months.
First-generation DES have an Achilles heel — stent thrombosis. According to recent data presented at the 2007 DES Summit, DES use for off-label indications is approximately 60 percent. Most of the off-label use is for the treatment of complex lesions, a subset of lesions conventional DES were never really designed to address. As a result, increased events of stent thrombosis have occurred.
However, stent thrombosis is not a new problem for DES. The first stent thrombosis red flag came in the form of a “Dear Doctor” letter around September 2004 from the FDA notifying physicians about the possible link between the off-label use of DES and acute stent thrombosis. The problem never really went away, and industry has been constantly reminded of the possibility of increased risks associated with the off-label use of the devices.
The Firestorm
Clearly, 2006 was not the year of the DES — particularly for DES manufacturers and those hopeful companies that dream of sharing a piece of the $6 billion DES market.
The rubber finally met the road mid-year at the European Society of Cardiology meeting in Barcelona, Spain, when Society members collectively announced that the use of DES increased the possibility of death and heart attacks. This announcement set off a firestorm surrounding the use of DES.
DES received the most flack toward the end of 2006, both from the medical community and the lay press. DES came to be referred to as “tiny ticking time bombs,” as a result of an NBC Nightly News special report by Robert Bazell that aired on Nov. 28.
The fact is, DES were never meant to cure CAD. Their purpose is to eliminate or significantly reduce restenosis, which, in most cases, is accomplished. Nevertheless, some interventional cardiologists were seduced by the early impressive angiographic results from the early DES trials, and today, some cardiologists even admit to having been lulled into a false sense of security by the early data.
The negative press has given new hope to the forlorn surgeons and noninvasive cardiologists, who, until recently, had been losing ground to interventional cardiologists because DES quickly captured the attention of CAD patients.
Now, according to Dr. Leon, more patients are being sent to bypass surgery and interventional cardiologists are implanting fewer stents than before. However, Dr. Leon does not think that this change in treatment is inappropriate.
“In any event I think that I listened very carefully and I'm trying to be much, much more thoughtful than in the early days of drug-eluting stents,” he said. “I think there's enough evidence for us to take notice and really consider how we should be treating patients.”
Back to CABG?
Perhaps bypass surgery, now more than ever, might be the preferred alternative to DES, in situations such as in multivessel CAD — this was proposed by Michael J. Mack, M.D., CRISTI Cardiopulmonary Research Science and Research Institute, a surgeon who was invited to present his “Perspectives from the Cardiothoracic Surgery Community” at the DES Summit.
Dr. Mack indicated that surgeons and interventionalists “are all trying to do the right thing but [they] are all biased.”
That is likely to be true. There are treatment biases based on a physician's practice area and referral preferences. There are clinical-trial biases based on inclusion and exclusion criteria. It is commonly known that device approval studies do not reflect real-world clinical practice. And, there are other procedure biases to think about.
In fact, it is not until the device is finally marketed, tracked and followed in post-market surveillance studies that the real data surfaces. This is what has happened with DES, similar to what has happened with blockbuster drugs and other medical therapies in the past.
The bottom line is: It is up to the FDA to take action and to mandate that manufacturers conduct more aggressive post-market studies. Perhaps then issues such as stent thrombosis could be caught and addressed before they become centerstage for a media circus.
Addressing the ST Issue
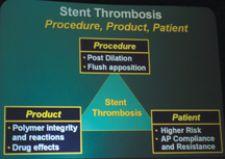
The causes of DES stent thrombosis are multifactorial, according to Dr. Cannon. This is supported by data presented at the DES Summit, which indicated that, in many cases, how the stent is deployed and whether patients adhere to their anticoagulation regimens can play a role in increasing thrombotic events. Roxana Mehran, M.D., NewYork-Presbyterian/
Columbia, an associate professor of Medicine, indicated that the polymer, the drug, the procedure itself and patient compliance all contribute to the occurrence of thrombotic events.
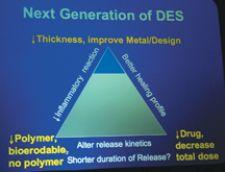
In fact, the issues surrounding first- generation DES polymers and drugs have been debated many times over the last two years. The consensus: Find a better polymer and the selection of less toxic drugs.
According to data presented by Renu Virmani, M.D., CVPath International Registry of Pathology, next-generation stents that have adopted this mantra, such as the Endeavor (Medtronic) and Xience V (Abbott), have shown promising results in rabbit iliac models with regards to re-endothelialization.
Dr. Virmani pointed to four specific reasons why she believes these newer generation stents show more promise: (1.) different release kinetics, (2.) improved drugs that can be applied in lower dosages, (3.) different and less inflammatory polymer coatings and (4.) quicker endothelialization. Dr. Virmani presented animal model data demonstrating better re-endothelialization for the Endeavor and Xience V stents compared to Cypher and Taxus at 28 days.
The Next Step
Boston Scientific is taking a proactive approach to educate physicians about the importance of using DES safely. According to Jeff Mirviss, Boston Scientific's vice president of Marketing for the global drug-eluting stent franchise, the company has become very transparent in terms of making available all the data they have about the TAXUS DES program to physicians and interested parties. Mirviss said that BSC wants physicians to be able to make informed decisions about DES. BSC is trying to send a clear and explicit message to the market that addressing the current DES safety concerns is a top priority.
Mirviss agrees with the sentiment that stent thrombosis is a multifactorial problem that requires a multifactorial solution. This is why BSC not only believes that it is important to “optimize the implant” by revisiting its current technologies (stents and release mechanisms), exploring better ways of delivering drugs (e.g., ablumenal delivery) and reevaluating the amount of polymer that is needed (e.g., thinner polymer layers), but the company also believes it is important to consider other factors, such as stent technique and placement strategy when designing new iterations of the technology. According to Mirviss, BSC has a full product pipeline, because the company is constantly working to improve its product offerings.
“We have a robust DES pipeline because each DES team builds on the previous team's work,” he said.
Mirviss pointed to their new Liberté stent, for example, which has thinner struts than the Express stent (0.0038” vs. 0.0052”). The design and geometry is a more uniform pattern of repeating elements, which Mirviss indicated “aids in making the stent more deliverable, while providing for homogeneous drug delivery.” According to Mirviss, BSC will also be initiating the PERSEUS trial in mid-2007 (for which Dr. Cannon is the trial's principal investigator) to test a new a hybrid stent material called Platinum Enriched Stainless Steel (or PERSE). The new material will be used in future BSC DES platforms. The PERSE material provides increased radiopacity and strength, while remaining extremely flexible and providing a balance between metal and design.
Mirviss indicated that BSC plans to use the new PERSE stent platform for its new PROMUS Element and TAXUS Element DES.




 July 02, 2024
July 02, 2024 









