
Promus Premier DES Receives European Approval
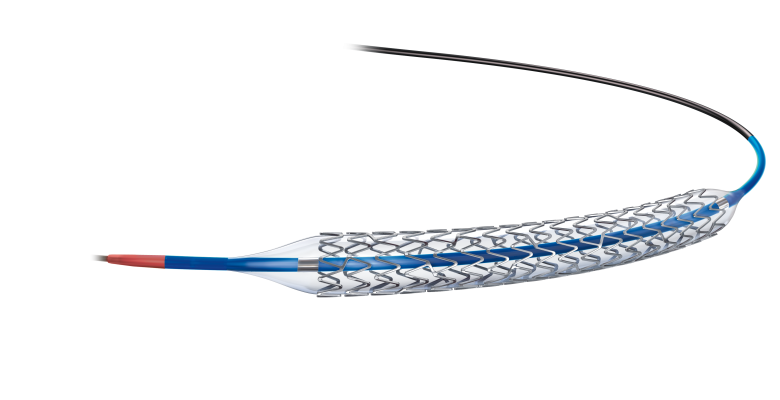
February 18, 2013 — Boston Scientific received CE mark approval for the Promus Premier everolimus-eluting platinum chromium coronary stent system, the company's next-generation durable polymer drug-eluting stent (DES) technology. The Promus Premier is designed to improve DES performance with a new delivery system and redesigned stent architecture that improves radial, axial strength and deliverability.
The new stent uses the same drug and polymer as the Promus Element, which Boston Scientific said compares very well in trials to the market leading competitor, Abbott’s Xience V. The company said the Promus Premier is being marketed as a workhorse stent.
Coronary heart disease is a narrowing of the vessels that supply blood and oxygen to the heart. Recent statistics from the European Heart Network and the European Society of Cardiology show it is the single most common cause of death in Europe accounting for 1.8 million deaths in Europe per year. Patients living with coronary heart disease, also known as coronary artery disease, may experience pain, shortness of breath and fatigue. They may also be at risk for a heart attack. One treatment option is the placement of a stent in the artery to help keep it open and allow the blood to flow more freely.
"The customized platinum chromium stent architecture maintains the superior visibility, exceptional radial strength and fracture resistance, minimal recoil and outstanding clinical outcomes of a PtCr everolimus-eluting stent while offering improved longitudinal strength," said John Ormiston, M.D., Mercy Angiography, Auckland City, New Zealand. "In addition, the enhanced stent delivery system offers improved deliverability. Stent design is a balance of trade-offs and the Promus Premier Stent System appears to have it right."
The Promus Premier Stent System was developed with input from physicians. The customized platinum chromium alloy stent architecture provides strength without compromising flexibility. An enhanced low-profile delivery system features a shorter, more visible tip, dual-layer balloon and bi-segment inner lumen catheter designed to facilitate precise stent delivery across challenging lesions. The Everolimus drug and fluorinated co-polymer stent coating have been studied in multiple randomized clinical trials demonstrating long-term safety and efficacy. The Platinum Workhorse Trial compared the Promus Element stent (platinum chromium everolimus-eluting stent) to the Xience V stent (cobalt chromium everolimus-eluting stent), and demonstrated that platinum chromium technology has superior clinical outcomes between years one and two.
The Promus Premier Stent System is currently offered in a matrix of 47 sizes, ranging in diameter from 2.25 to 4 mm and lengths of 8 to 38 mm on a monorail catheter platform. This comprehensive offering provides cardiologists and their patients a broad range of options designed to best suit their needs. The Promus Premier Stent System is not available for sale in the United States or Japan.
For more information: www.bostonscientific.com


 July 02, 2024
July 02, 2024 









