
Gadolinium-enhanced MRI of a cardiac radiation therapy patient at baseline (left) and 3 months post-treatment (right). Top: the left ventricle with patchy, gadolinium-enhanced scar was transmurally targeted with a radiation ose of 25 Gy between 3 and 6 o’clock (red brackets). Nonenhanced, remote myocardium is adjacent to target region (white arrowhead). Bottom: surviving nonenhanced myocardium within the same images is visible in the targeted region at baseline and 3 months post-treatment (yellow outline). See more figures from this study.
September 28, 2021 — New research from Washington University School of Medicine in St. Louis suggests that radiation therapy can reprogram heart muscle cells to what appears to be a younger state. Researchers say this may fix electrical problems that cause a life-threatening arrhythmias like ventricular tachycardia without the need for the current standard of care, invasive ablation procedures.[1]
In electrophysiology (EP) catheter ablation, tissue that triggers ventricular tachycardia is burned, creating scars that block the errant signals. The new study, however, shows that noninvasive radiation therapy normally used to treat cancer can reprogram the heart muscle cells to a younger and perhaps healthier state, fixing the electrical problem in the cells themselves without needing scar tissue to block the overactive circuits. The study also suggests that the same cellular reprogramming effect could be achieved with lower doses of radiation, opening the door to the possibility of wider uses for radiation therapy in different types of cardiac arrhythmias.
The study "Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis" appears Sept. 24 in the journal Nature Communications.[1]
Physician-scientists at Washington University showed in 2017 that radiation therapy typically reserved for cancer treatment could be directed at the heart to treat ventricular tachycardia.
In theory, radiation therapy could reproduce the scar tissue usually created through catheter ablation but with a much shorter and totally noninvasive procedure, making the treatment available to more severely ill patients. Surprisingly, the doctors found that patients experienced large improvements in their arrhythmias a few days to weeks after radiation therapy, much quicker than the months it can take scar tissue to form after radiation therapy, suggesting that a single dose of radiation reduces the arrhythmia without forming scar tissue. The data indicated that radiation treatment worked just as well, if not better, than catheter ablation for certain patients with ventricular tachycardia but in a different and unknown way.
“Traditionally, catheter ablation creates scar tissue to block the electrical circuits that are causing ventricular tachycardia,” said senior author and cardiologist Stacey L. Rentschler, M.D., Ph.D., an associate professor of medicine, of developmental biology and of biomedical engineering. “To help us understand whether the same thing was happening with radiation therapy, some of the first patients to have this new treatment gave us permission to study their heart tissue — following heart transplantation or if they had passed away for another reason, for example. We saw that scar tissue alone could not explain the remarkable clinical effects, suggesting that radiation improves the arrhythmia in some other way, so we delved into the details of that.”
The scientists found that radiation treatment triggered heart muscle cells to begin expressing different genes. They measured increased activity in a signaling pathway called notch, which is known for its vital role in early development, including in forming the heart’s electrical conduction system.
Notch is usually switched off in adult heart muscle cells. But the researchers found that a single dose of radiation temporarily activates notch signaling, leading to a long-term increase in sodium ion channels in the heart muscle, a key physiologic change that can reduce arrhythmias.
“Arrhythmias are associated with slow electrical conduction speeds,” Rentschler said. “Radiation therapy seems to kick up the speed faster by activating early developmental pathways that revert the heart tissue back into a healthier state.”
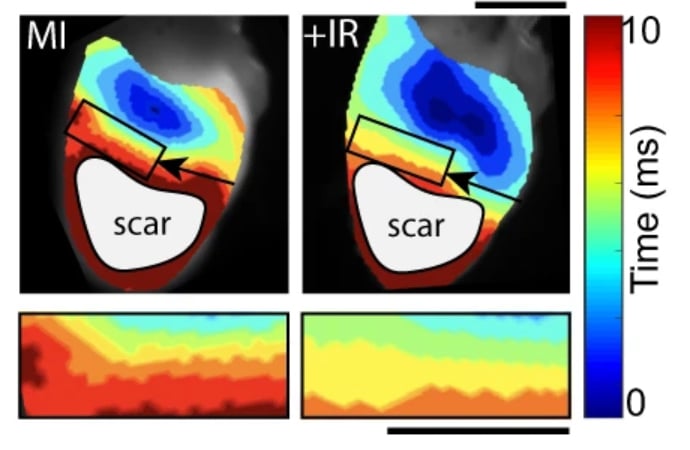
Representative ventricular activation maps from control myocardial infarction (MI, left) and irradiated MI (MI+IR, right) mice at 8 weeks post-MI and 6 weeks post-IR. Black arrows point to location of left anterior descending (LAD) artery ligation. Black box denotes approximate 2 mm border zone at the scar/myocardium interface. Regions of scar that were epicardially stimulated, but did not capture are denoted by gray circles. Magnified isochrones in the border zone region used to calculate transverse conduction velocities. See more figures in this study.
The researchers studied these effects in mice and in donated human hearts. In the human heart samples, the researchers found that these changes in heart muscle cells were only present in areas of the heart that received the targeted radiation dose.
“Radiation does cause a type of injury, but it’s different from catheter ablation,” said co-author and radiation oncologist Julie K. Schwarz, M.D., Ph.D., a professor of radiation oncology and director of the Cancer Biology Division in the Department of Radiation Oncology. “As part of the body’s response to that injury, cells in the injured portion of the heart appear to turn on some of these early developmental programs to repair themselves. It’s important to understand how this works because, with that knowledge, we can improve the way we’re treating these patients and then apply it to other diseases.”
The researchers also found that the beneficial effects of radiation continued for at least two years in surviving patients. And importantly, they were able to demonstrate in mice that a lower dose of the radiation produced the same effect. A lower radiation dose could minimize long-term side effects and open the door to this type of treatment in other types of heart arrhythmias. And while Notch was a big player in these effects, Schwarz said it’s not the only pathway involved. The researchers are continuing to investigate how radiation triggers heart cells to revert to a healthier state.
“This was an exciting collaboration not only between basic scientists and clinicians but also cardiologists and radiation oncologists. Historically, radiation oncologists are focused on cancer and try to avoid irradiating the heart, so this study opens up a whole new area of research and collaboration between these two fields,” aded first author David M. Zhang, an M.D./Ph.D. student in Rentschler’s lab.
This work was supported by the National Institutes of Health (NIH), grant numbers T32 HL134635, T32 GM07200, R01 HL130212, UH3 HL141800 and S10 OD020136. This study received seed funding from the Department of Radiation Oncology, Cancer Biology Division, at Washington University.
Schwarz holds a Female Investigator Award from AACR-Bristol Meyers Squibb and funding from the Radiological Society of North America. Rentschler holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund and funding from The Foundation for Barnes-Jewish Hospital that directly supported this work.
Washington University co-authors Clifford Robinson, M.D., and Phillip Cuculich, M.D., have filed two institution-owned patents: Noninvasive Imaging and Treatment System for Cardiac Arrhythmias that relates to overall methods for delivery of cardiac radiation in patient; and System and Method for Determining Segments for Ablation that relates to use of cardiac segments for cardiac radiation targeting. They also provide consulting services to Varian, which produces linear accelerators for radiation treatment delivery.
Related Radiotherapy for Cardiac Ablation Content:
Radiation Therapy to Treat Heart Arrhythmias is a Technology to Watch
New Technologies to Improve Atrial Fibrillation Ablation
New Targets for Radiation Therapy Include Cardiac AF Ablations and Renal Denervation
VIDEO: Using Radiosurgery for Atrial Fibrillation Cardiac Ablation
CyberHeart to Develop Noninvasive Cardiac Ablation Technique Using Accuray Robotic System
VIDEO: Cardiac Radiotherapy Ablation to Treat Ventricular Tachycardia — Interview with Clifford Robinson, M.D.
VIDEO: Use of Radiotherapy to Noninvasively Ablate Ventricular Tachycardia — Interview with Pierre Qian, MBBS
Varian Acquires CyberHeart Cardiac Radio-ablation Technology
Noninvasive Radioablation Offers Long-term Benefits to High-risk Heart Arrhythmia Patients
Find more radiation oncology news
Reference:


 January 05, 2026
January 05, 2026 









