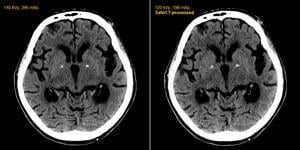
Left: standard-dose image at 396 mAs, 140 kVp. Right: SafeCT reconstructed image from the same patient imaged at 198 mAs, 120 kVp. The software offers a similar image with 50 percent less dose.
Computed tomography (CT) offers a very important, noninvasive diagnostic tool, but the price of high image quality sometimes comes with a cost of high radiation dose. This is especially true of CT angiography (CTA), which may require imaging several cardiac cycles. CT scans have been a trade-off between good images using higher doses, or lower image quality using lower doses. Advancements in CT imaging processing software have helped improve the image quality from low-dose scans by eliminating noise. This can help lower patient radiation dose by 30-80 percent, according to the manufacturers, without compromising diagnostic image quality. The primary technique uses iterative image reconstruction. Early versions of the software were slow and required much more time to produce a final image than simply using a higher dose. However, advancements have greatly reduced the processing time. High-dose CT scans were brought into the public eye last year with mainstream media attention. With radiology associations and the U.S. Food and Drug Administration (FDA) proposing radiation dose monitoring, registries and procedural limits, dose-lowering technology will play an increasingly important role. CT Manufacturers During the 2010 Radiological Society of North America (RSNA) meeting in December, all the major CT vendors introduced new dose-lowering software or highlighted works-in-progress. Philips introduced its Ingenuity CT, which features the company’s new iDose4 iterative reconstruction software. It is designed to provide equivalent diagnostic image quality at up to 80 percent less dose. It can also improve spatial resolution up to 35 percent with up to 50 percent less dose. The new platform is available as a standalone or as a hybrid molecular imaging system. “In the past, because the combination of great image quality, low dose and fast reconstruction times has been a challenge for the industry, CT scanning has often been about trade-off,” said Gene Saragnese
, Philips imaging systems CEO. However, he said the new system allows both low dose and fast image reconstruction. AT RSNA, Siemens previewed its FAST CARE (Fully Assisting Scanner Technologies - Combined Applications to Reduce Exposure) CT application to assist with dose reduction and faster workflow. It automates many operating procedures, suggests parameter settings for image quality and dose reduction, and standardizes processes. FAST CARE will be available on the Somatom Definition AS scanners in March 2011 and on the Somatom Definition Flash scanners in May 2011. Installed Definition family CT scanners can be upgraded to the new platform. Toshiba showed its works-in-progress Adaptive Iterative Dose Reduction (AIDR) technology, which iterates noise to increase quality and lower radiation dose by up to 75 percent. The software intuitively improves the image by removing noise until the optimal image is produced. AIDR is pending 510(k) clearance, but will be standard on the Aquilion Premium and Aquilion One. GE Healthcare showcased its ASiR (adaptive statistical iterative reconstruction) low-dose reconstruction technology, which is available on the GE Discovery CT750 HD and LightSpeed VCT. The company says it reduces dose by up to 40-50 percent while maintaining image quality and can be implemented as a cost-effective upgrade for existing LightSpeed VCT systems. The technology can be used on both helical and axial scans to reduce dose and maintain image quality for patients of all ages. ASiR is now installed on more than 600 CT scanners worldwide. GE’s newest low-dose technology is a model-based iterative reconstruction (MBIR) technology called Veo. It is currently available in Europe and is pending 510(k) clearance in the United States. When compared to previous GE reconstruction methods, Veo’s new modeling techniques deliver resolution gain, lower noise, improved low-contrast detectability and artifact suppression. Third-Party Vendors In January, Medic Vision received FDA clearance to market its add-on SafeCT product in the United States. The software processes and enhances images on a wide variety of CT scanners. SafeCT enables a 50 percent dose reduction in CT scans without compromising image quality and diagnostic information. SafeCT, which is compatible with most major CT platforms and picture archiving and communication systems (PACS), can provide low-dose imaging functionality to these scanners. “We are exploring strategic partnerships to facilitate the distribution of our CT dose-reduction system to American clinicians and to promote synergetic collaborations for integrating our system into existing and emerging CT scanner platforms,” said Medic Vision CEO Eyal Aharon. ContextVision offers OEM image processing solutions as an add-on for CT manufacturers. Its GOPView CT image enhancement software operates on a standard CPU. It can reduce image noise while enhancing fine structures and edges at both standard and low-dose images. The company says clinical studies and current installations demonstrate exceptional image quality can be achieved in images with a 30-70 percent lower dose. The GOP algorithm is used by OEMs to enhance fine structures and reduce noise across magnetic resonance imaging (MRI), X-ray, ultrasound and CT image data.


 February 02, 2026
February 02, 2026 









