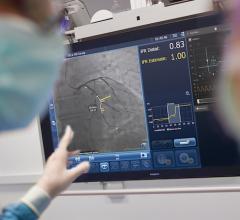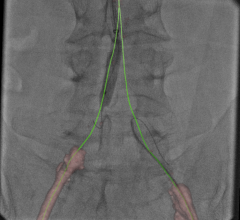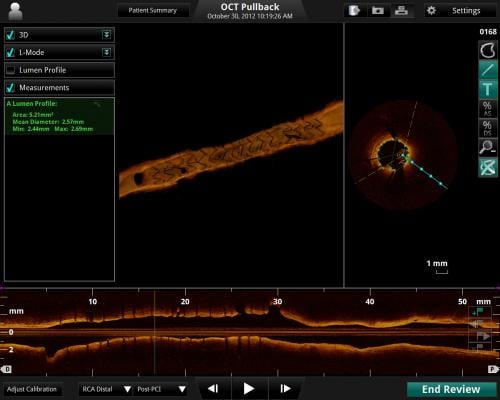
May 16, 2013 — St. Jude Medical gained CE mark approval of its Ilumien Optis percutaneous coronary intervention (PCI) optimization system to better visualize stent planning and navigation. The system will be on display for the first time in Europe during EuroPCR.
The Ilumien Optis system provides enhancements to the Ilumien system, including first-of-its-kind stent planning software tools to aid in the treatment of coronary artery disease (CAD). The Ilumien platform integrates both fractional flow reserve (FFR) technology to measure pressure inside the coronary arteries and intravascular optical coherence tomography (OCT) imaging technology, in one system.
Featuring a faster, high-powered laser, the Ilumien Optis system offers twice the resolution for microscopic examination of disease inside the artery to assist with stent placement. The real-time, 3-D reconstruction offers a 360-degree panoramic view of the vessel, making it easier for physicians to visualize the area they are treating. St. Jude Medical is the only company to provide these tools together in an integrated platform.
“OCT technology has become increasingly important to help diagnose and treat patients with coronary artery disease. The Ilumien Optis system is a significant advancement in intravascular imaging technology allowing physicians to comprehensively assess more vessel in less time and more easily plan their PCI procedure. The three-dimensional format of the Ilumien Optis system provides a more true-to-life perspective of the arteries, which allows for individual decision making and precise guidance of stent placement to optimize coronary interventions, said Dr. Giulio Guagliumi, cardiovascular department of Ospedale Papa Giovanni XXIII, Bergamo, Italy.
The OCT technology in the new Ilumien Optis system uses the Dragonfly Duo Imaging Catheter to capture near-infrared light imaging and measure important vessel characteristics otherwise invisible or difficult to assess with older imaging technology. When used with the system, the Dragonfly Duo catheter offers faster, longer pull-backs, which allow the physician to assess more of the patient’s artery in less time.
The wireless PressureWire Aeris technology that is integrated into the platform measures pressure differences in blood flow within the coronary arteries leading to the heart, and determines the severity of any narrowings or blockages. The FFR pressure guidewire is directed along the vessel, taking measurements as the guidewire is pulled back through the artery. Knowing which specific blockages are causing the patient’s blood flow to be ineffective helps guide the interventional cardiologist in determining which lesions warrant stenting, resulting in improved patient outcomes and reduced health care costs.
The FFR and OCT measurements captured by the Ilumien Optis system allow physicians to more easily differentiate plaque build-up and determine if the narrowed arteries are causing ischemia, ultimately assisting in stent placement. The automated stent planning tools provide immediate information for assessment and real-time analysis, which is intended to streamline workflow, potentially helping physicians diagnose their patients more quickly.
For more information: sjm.com

 January 18, 2022
January 18, 2022