
March 29, 2012 — Patients who underwent transcatheter aortic valve implantation (TAVI) at experienced medical centers had significant improvement in valve function as well as low mortality and stroke rates at 30 days, according to research presented this week at the American College of Cardiology (ACC) Annual Scientific Session.
The TAVI procedure includes inserting a bioprosthetic valve through a catheter and implanting it into a diseased native aortic valve. Because it is less invasive than surgical replacement, TAVI has been used for patients with a diseased aortic valve who cannot undergo surgery because of age, left ventricular dysfunction or the presence of coexisting conditions.
The ADVANCE study follows several trials already conducted to determine the safety and effectiveness of TAVI; however, it is the first large TAVI trial to use the Valve Academic Research Consortium’s (VARC) standards to define adverse events. It also used 44 centers that had each performed at least 40 TAVI procedures before joining the trial.
“This study evaluated real-world patients in everyday practice, allowing us to better understand the outcomes of typical use of TAVI,” said lead study author Axel Linke, professor of cardiology at the University of Leipzig Heart Center in Leipzig, Germany. “In addition, this study is one of the most rigorous TAVI studies ever conducted, and it is the first, large multicenter TAVI trial to report findings according to the new standard of evaluation, VARC.”
Between March 2010 and July 2011, a total of 1,015 high-risk patients with severe aortic stenosis were enrolled at 44 centers in 12 countries in Western Europe, Asia and South America. The patients’ mean age was 81 ± six years and 51 percent were female. The patients underwent TAVI and received the CoreValve System. They were then followed to determine the safety and efficacy of the procedure.
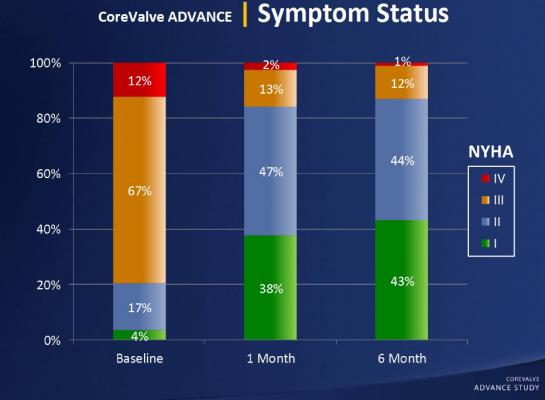
The study’s primary endpoint was the occurrence of major adverse cardiac and cerebrovascular events (MACCE), which is a composite of total death, stroke, heart attack and emergent cardiac surgery or percutaneous reintervention. The efficacy of the procedure was also analyzed by measuring valve function.
At the 30-day follow-up point, the researchers found that MACCE occurred in 8.3 percent of patients. The rates of total mortality and cardiac mortality were 4.5 percent and 2.2 percent, respectively, and the stroke rate was 2.9 percent. Life-threatening or disabling bleeding was observed in 4 percent of patients. The research team also found a persistent improvement in aortic valve function, accompanied by a better exercise capacity.
“The study’s conclusion is straightforward — that TAVI is effective and is associated with low mortality in the presence of an improvement in symptoms,” said Linke. “We included patients that were considered high operative risk and non-operable, and they definitely showed symptom improvement. The procedure provided great relief for the patients, as they did much better after implantation.”
The study was funded by Medtronic Inc. Linke disclosed that he is a consultant to Medtronic.
For more information: www.acc.org



 January 05, 2026
January 05, 2026 









