
A prototype bioresorbable zinc stent developed by the University of Michigan.
May 15, 2013 — In 2012, more than 3 million people had stents inserted in their coronary arteries. After about six months, most damaged arteries are healed and stay open on their own. The stent, however, is there for a lifetime, which can cause negative side effects, leading to the development of bioresorbable stents.
Most of the time a conventional stent is not a problem, says Patrick Bowen, a doctoral student studying materials science and engineering at Michigan Technological University. The arterial wall heals in around the old stent with no ill effect. But the longer a stent is in the body, the greater the risk of late-stage side effects. For example, a permanent stent can cause intermittent inflammation and clotting at the implant site. In a small percentage of cases, the tiny metal segments that make up the stent can break and end up poking the arterial wall.
“When the stent stays in place 15, 20 or 25 years, you can see these side effects,” said Bowen. “It’s not uncommon to have a stent put in at age 60, and if you live to be 80, that’s a long time for something to remain inert in your body.”
That’s why researchers are trying to develop a bioabsorbable stent, one that would gradually — and harmlessly — dissolve after the blood vessel is healed.
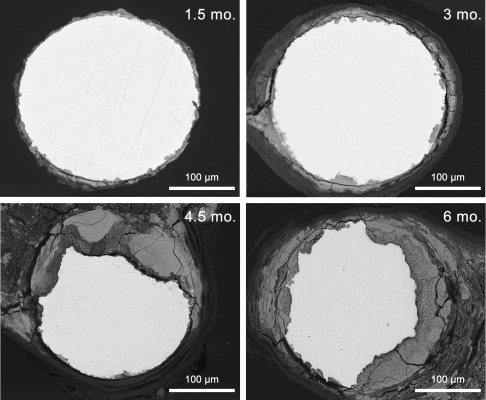
Many studies have investigated iron- and magnesium-based stents. However, iron is not promising: it rusts in the artery. Magnesium, on the other hand, dissolves too quickly. “We wondered, ‘Isn’t there something else?’” Bowen said. “And we thought, ‘Why not zinc?’”
So they placed tiny zinc wires in the arteries of rats. The researchers said the results were amazing. “The corrosion rate was exactly where it needed to be,” Bowen said. The wires degraded at a rate just below 0.2 millimeters per year — the “magic” value for bioabsorbable stents — for the first three months. After that, the corrosion accelerated, so the implant would not remain in the artery for too long. On top of that, the rats’ arteries appeared healthy when the wires were removed, with tissue firmly grasping the implant.
“Plus, zinc reduces atherosclerosis,” he added, referring to zinc’s well-known ability to fight the development of plaque in the arteries. “How cool is that? A zinc stent might actually have health benefits.”
However, researchers said there is one drawback. “A stent made of conventional zinc would not be strong enough to hold open a human artery,” he said. “We need to beef it up, double the strength.”
“The good news is that there are commercial zinc alloys that are up to three times stronger,” Bowen said. “We know we can get there. We just don’t want to ruin our corrosion behavior.”
The researchers have filed a provisional patent on their discoveries and are now testing new zinc-based stent materials.
An article on their work, “Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents,” was recently published May 14 in the journal Advanced Materials.
Bowen’s research is supported by a two-year, $52,000 predoctoral fellowship from the American Heart Association. Initial research was supported by a summer fellowship from the DeVlieg Foundation. Bowen’s advisor is Jaroslaw Drelich, a professor of materials science and engineering, and they work in close collaboration with Jeremy Goldman, an associate professor of biomedical engineering.
For more information: www.mtu.edu/news/


 January 05, 2026
January 05, 2026 









