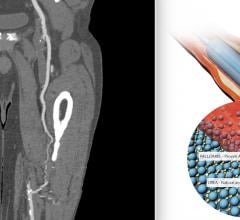
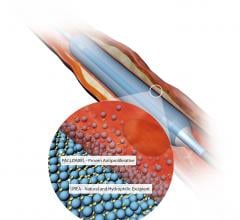
October 20, 2021 — 18-month results from the PRESTIGE* Below-the-Knee (BTK) study have been presented as a late-breaking ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
June 7, 2021 — A couple years ago a study showed a mortality safety signal in patients who underwent peripheral artery ...
May 20, 2021 — Boston Scientific Corporation announced it has initiated the AGENT IDE trial for the Agent Drug-Coated ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
This is a quick example of clinical use of the Shockwave Medical Intravascular Lithotripsy system that uses sonic wave ...
After covering cardiovascular technologies for 14 years, it is rare that I find a new technology that might actually be ...

The first devices developed for interventional cardiology were percutaneous transluminal coronary angioplasty (PTCA) ...
March 12, 2021 — Philips Healthcare announced the final, five-year results of two major randomized controlled trials ...

February 17, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Shockwave Medical's Intravascular ...
November 13, 2020 — The new U.S. Food and Drug Administration (FDA) cleared XO Score system was successfully used to ...
November 3, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the Boston Scientific Ranger Drug-coated ...

October 18, 2020 – A large subgroup analysis of a randomized clinical trial showed neither a mortality risk nor benefit ...
October 18, 2020 – The first results from the IN.PACT Below the Knee (BTK) Study, a feasibility study assessing the ...
Juan F. Granada, M.D., CEO of the Cardiovascular Research Foundation (CRF) worked on preclinical development work for a ...
September 8, 2020 — Researchers developed a new class of medical instruments equipped with an advanced soft electronics ...

Drug-coated balloons (DCB), also referred to as drug-eluting balloons (DEB), were created as a way to reduce very high ...


 October 20, 2021
October 20, 2021