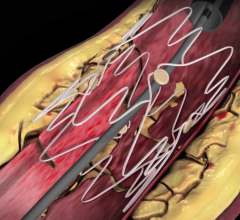
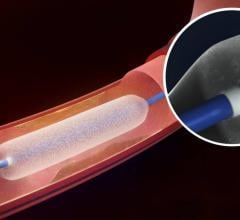
July 18, 2016 — Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for the IN.PACT Admiral drug ...
Balloon Catheter
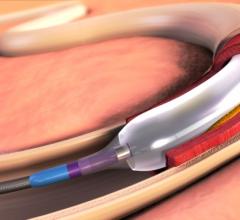
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
June 3, 2016 — Cardionovum GmbH recently announced the completion of enrollment of the RAPID trial. Results will be used ...
May 5, 2016 — Intact Vascular Inc. announced that positive twelve-month results from its Tack Optimized Balloon ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
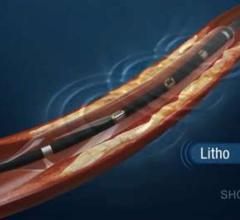
March 28, 2016 — NuCryo Vascular LLC announced the launch of the Next Generation Cryoplasty Inflation device. The device ...
March 22, 2016 — PinnacleHealth CardioVascular Institute enrolled the first patient in Pennsylvania into the TOBA II ...
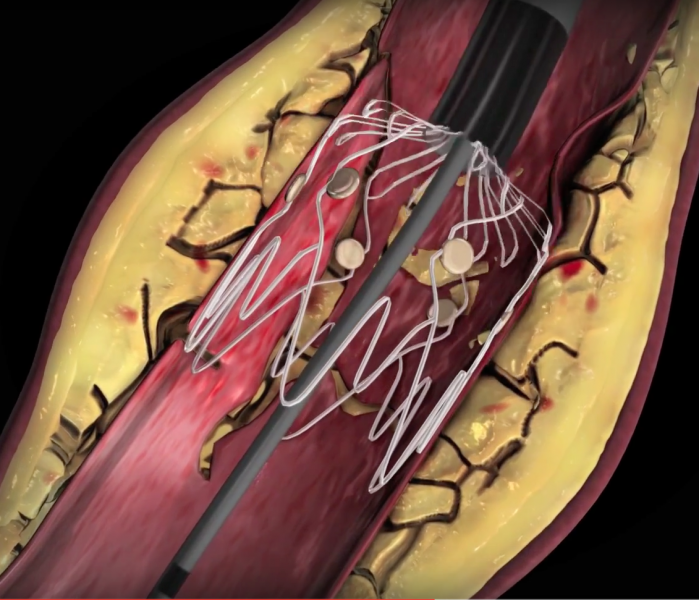
It is estimated that more than 10 million people in the United States are affected by peripheral arterial disease (PAD) ...
January 29, 2016 — Intact Vascular Inc. announced that positive six-month results from its Tack Optimized Balloon ...
January 18, 2016 — Medtronic plc announced that the IN.PACT Admiral drug eluting balloon (DEB) has received CE ...
January 12, 2016 — NuCryo Vascular LLC announced that they have received U.S. Food and Drug Administration (FDA) 510(k) ...
November 24, 2015 — The U.S. Food and Drug Administration (FDA) issued a warning this week that hydrophilic and/or ...
Todd Brinton, M.D., clinical associate professor and consulting associate professor of bioengineering at Stanford ...

October 22, 2015 — Results from the ISAR-DESIRE 4 trial indicate that use of a scoring balloon plus a paclitaxel-coated ...

October 18, 2015 — Two-year results from the IN.PACT SFA study found the use of a drug-coated balloon was superior to co ...
October 15, 2015 — C. R. Bard Inc. announced the presentation of the 12-month results from the Lutonix Global Real-World ...
October 9, 2015 — Cardinal Health is launching its expanded portfolio in the cardiovascular space including the latest ...


 July 18, 2016
July 18, 2016