October 30, 2015 — Boston Scientific Corp. has received CE Mark on magnetic resonance imaging (MRI) conditional labeling ...
Cardiac Resynchronization Therapy Devices (CRT)
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
September 3, 2015 — Sorin Group announced the start of patient enrollment in its TRIUMPH-CRT clinical trial in Europe.
...
September 3, 2015 — Most patients with a cardiac resynchronization therapy (CRT) pacemaker would not benefit from the ...
August 17, 2015 — Biotronik announced that the first patient has been enrolled in the BioCONTINUE clinical trial ...

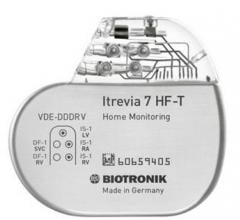
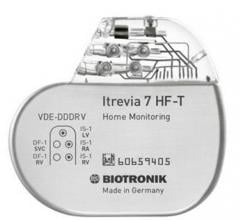
July 28, 2015 — Biotronik announced the first patients have been successfully implanted with Itrevia HF-T QP cardiac ...
July 22, 2015 — Heart failure patients had a significantly lower chance of being readmitted within 30 days of discharge ...
May 19, 2015 — A new epicardial pacing lead has been cleared by the U.S. Food and Drug Administration (FDA) as an option ...

May 15, 2015 — A new expert consensus statement by the Heart Rhythm Society (HRS) recommends remote monitoring (RM) beco ...
May 5, 2015 — Biotronik announced that a unique physiologic sensor is now available in the latest family of cardiac ...
April 17, 2015 — EBR Systems Inc. announced it has raised $20 million for clinical studies and regulatory filings ...
Learn about the trends and new technology in electrophysiology (EP) in an interview with David Wilber, M.D., editor of ...
January 30, 2015 — Medtronic announced new results from the PainFree SST and Shock-Less clinical studies published in ...
September 10, 2014 — Medtronic Inc. announced the first implants in a clinical trial that will compare patient and ...
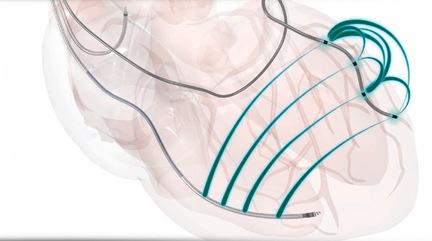
September 9, 2014 — Use of a quadripolar left ventricular (LV) lead instead of a bipolar option during cardiac ...
August 28, 2014 — Medtronic Inc. announced the U.S. Food and Drug Administration (FDA) approval of its newest cardiac ...

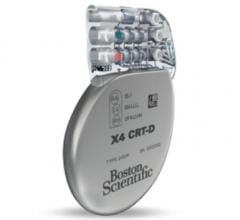
 October 30, 2015
October 30, 2015