July 16, 2012 — The U.S. Food and Drug Administration (FDA) notified healthcare professionals of a Class I recall of ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 13, 2012 — Angioslide Ltd., a provider of embolic capture angioplasty solutions, today announced the successful ...
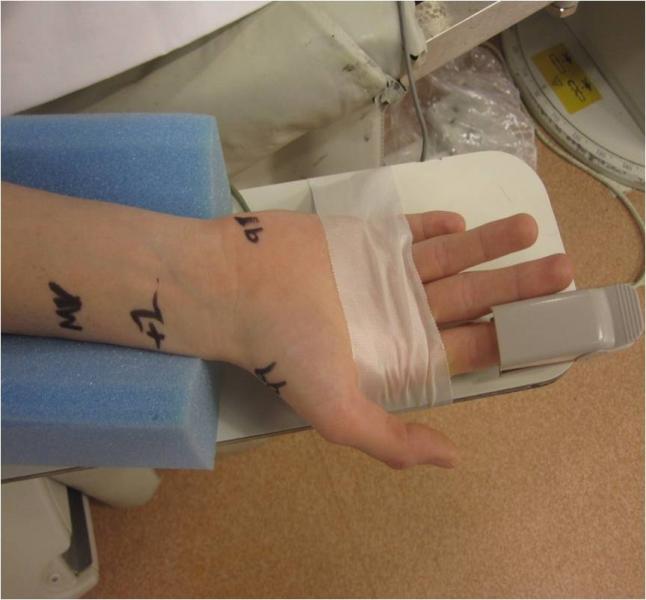
July 13, 2012 — In the United States, radial artery catheterization is performed in the minority of diagnostic ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

July 13, 2012 ? An otherwise healthy pregnant woman who suffered cardiac arrest and was resuscitated, therapeutically ...
July 12, 2012 — Terumo Interventional Systems, a strategic business unit of Terumo Medical Corporation, a U.S ...
July 11, 2012 — Ultrasonix Medical Corporation has received approval from the U.S. Food and Drug Administration (FDA) ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
July 9, 2012 — r4 Vascular Inc. announced clearance from the U.S. Food and Drug Administration (FDA) to market the ...
July 9, 2012 — Miracor Medical Systems GmbH announced that the first three ST segment elevation myocardial infarction ...
July 9, 2012 — Cardionovum GmbH announced it has initiated in vivo testing of its second drug-eluting balloon (DEB) Rest ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
July 9, 2012 — Burlington Medical Supplies, a radiation protection and X-ray medical supply company, now offers no fog ...
July 6, 2012 — Vascular Closure Systems Inc. announced that the 30-day follow-up results for the Phase I first-in-human ...

July 6, 2012 — With last year’s U.S. Food and Drug Administration approval of transcatheter aortic valve replacement ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Three big trends were seen in cardiovascular ultrasound during the American Society of Echocardiography (ASE) 2012 ...
July 5, 2012 — Avinger Inc., a medical device manufacturer of multifunctional catheters for treating patients with ...
July 3, 2012 — Flexible Stenting Solutions Inc., a leading developer of next generation peripheral arterial, venous ...


 July 16, 2012
July 16, 2012