After several years of trials and increasing clinical use, transcatheter aortic valve replacement (TAVR) has reached a ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
September 8, 2014 — The U.S, Food and Drug Administration (FDA) has cleared MicroVention Inc.’s Low-Profile Visualized ...
September 5, 2014 — The number of uninsured is expected to decline by nearly half from 45 million in 2012 to 23 million ...
September 5, 2014 — CardiacAssist announced it has received 510(k) clearance from the U.S. Food and Drug Administration ...
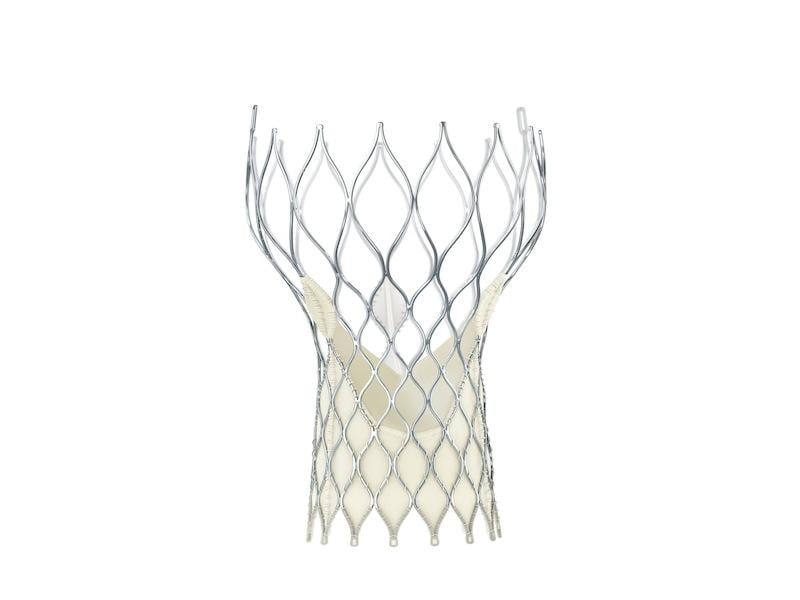
September 4, 2014 — Medtronic announced CE mark for the 23 mm CoreValve Evolut R system for transcatheter aortic valve ...
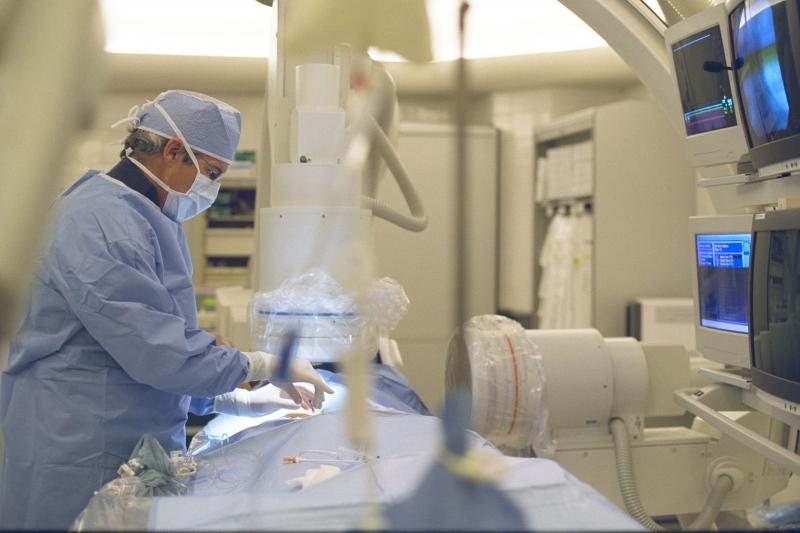
September 4, 2014 – For the first time in the United States, doctors at Henry Ford Hospital used a minimally invasive ...
September 3, 2014 — Teleflex announced a newly published clinical study demonstrating the accuracy of the Arrow VPS G4 ...
September 2, 2014 — Philips has launched Affiniti. The new ultrasound system made its debut at the European Society of ...
September 2, 2014 — Boston Scientific has released the primary endpoint results from its NEural Cardiac TherApy foR ...
September 2, 2014 — Renal denervation reduces recurrent atrial fibrillation (AF) when performed with pulmonary vein ...
August 29, 2014 — The high demand for qualified health information technology (IT) professionals continues, as revealed ...
August 29, 2014 — Nuance Communications announced it has achieved an industry-first milestone in sharing 3 billion ...
August 29, 2014 — Direct Flow Medical announced it received CE mark for a 23 mm valve as part of its Direct Flow Medical ...
August 29, 2014 — Cook Medical recalled 696 of its CloverSnare four-loop vascular retrieval snare devices, model number ...

 September 08, 2014
September 08, 2014