August 25, 2008 - Vermillion Inc. said last week a study published in the August 2008 issue of the journal Vascular ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
August 11, 2008 - Flexible Stenting Solutions Inc. said today a physician at Auckland City Hospital in New Zealand ...
EKOS Corp. offers the EkoSonic Endovascular System (EkoSonic).
The design includes an advanced control unit with ...
AngioScore Inc. has introduced longer and larger AngioSculpt PTA Scoring Balloon Catheters for the treatment of ...
July 11, 2008 - AngioScore Inc. today announced the launch of new longer and larger AngioSculpt PTA Scoring Balloon ...
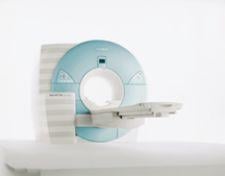
The only safe assumption to make about today’s current gold standard imaging modality used to identify and ...
July 9, 2008 - Summit Doppler Systems this week introduced a significant upgrade to the Vista AVS, a full-featured ...
June 16, 2008 - Datascope Corp. said it has exercised its option to acquire the Peripheral Vascular Stent business of ...
June 12, 2008 - Sorin Group closed the sale of its peripheral stent business to Datascope Corp., divesting its noncore ...
June 5, 2008 - Older patients may have less ability to repair of vessels and arteries after acute injury, similar ...
May 6, 2008 - Vascular Insights received 510(k) clearance from the FDA to market its ClariVein infusion catheter ...
The Society for Cardiovascular Angiography and Interventions (SCAI) has partnered with the American College of ...
March 3, 2008 - Vermillion Inc. has entered into an exclusive license agreement with Stanford University to develop and ...
February 7, 2008 - A Northwestern University Feinberg School of Medicine researcher has launched the first U.S ...
January 22, 2008 � Blocked abdomen and leg arteries, known as peripheral arterial disease (PAD), is more costly to ...

 August 24, 2008
August 24, 2008
