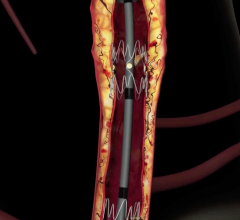
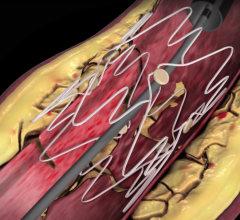
Feb. 1, 2007 — Cook Medical released nine-month data this week for its PTX Paclitaxel-Eluting Stent (DES) trial, which to date demonstrate no stent fractures. The major adverse event (MAE) rate was equivalent to conventional balloon angioplasty treatment at its six-month follow-up point, as reported by the trial's national principal investigator, Michael Dake, M.D., professor and chairman of the department of radiology at the University of Virginia Health System.
During the International Symposium on Endovascular Therapy (ISET), Dr. Dake, presented important nine-month data on the first 60 patients in the randomized trial examining the safety of using Cook's Zilver PTX stent to treat blockages of the superficial femoral artery (SFA) above the knee. He reported that the Zilver PTX stent displayed a zero-percent fracture rate for 41 lesions at six months and 18 lesions at one year.
It is the first trial ever to investigate whether Paclitaxel-eluting stents can be used to successfully treat peripheral arterial disease (PAD), a seriously under-diagnosed disorder affecting up to 20 percent of the adult population worldwide.
Effectiveness of the device in treating lesions of the SFA will be shown in the pivotal trial, expected to start shortly in the U.S., enrolling 420 patients at 50 sites.
This Cook's Zilver PTX drug-eluting vascular stent is an investigational device not approved for sale in the U.S., but under investigation for use in the treatment of symptomatic vascular disease of the above-the-knee femoropopliteal artery.
For more information visit www.zilverptxtrial.com or www.cookmedical.com.


 November 06, 2019
November 06, 2019