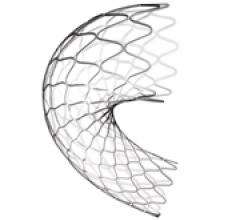
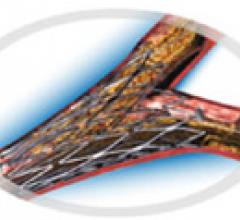
November 15, 2011 – A clinical trial that compared the use of drug-eluting balloons (DEB) and bare metal stents (BMS) to ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
November 15, 2011 — Results of ADAPT-DES (Assessment of Dual AntiPlatelet Therapy with Drug-Eluting Stents) were ...
November 15, 2011 — Researchers have found polymer-free amphilimus-eluting stents in de novo coronary artery lesions ...
November 15, 2011 — A new clinical trial is testing the efficacy of rotational atherectomy (or rotablation, a process of ...
November 15, 2011 — A clinical trial has shown a drug-eluting stent (DES) with a bioabsorbable polymer has comparable ...
November 14, 2011 – OrbusNeich announced the introduction of the Combo dual therapy stent during a breakfast symposium ...
November 14, 2011 – The TWENTE clinical trial, which compared Resolute versus Xience V drug-eluting stents in a real ...
November 11, 2011 – Boston Scientific reported results from the EVOLVE first human use trial demonstrating the non ...
November 11, 2011 – Biosensors International Group announced two-year results from the BioFreedom first-in-human trial ...
November 9, 2011 — Currently under review by the U.S. Food and Drug Administration (FDA), the Resolute drug-eluting ...
November 8, 2011 — Biosensors International Group Ltd. announced final results of the AXXESS PLUS trial, which ...
November 8, 2011 – Boston Scientific reports clinical endpoint data from its PLATINUM Long Lesion trial, demonstrating ...
November 7, 2011 – Abbott announced the company's schedule of key data presentations at the 23rd annual Transcatheter ...
Nov. 2, 2011 – The U.S. Food and Drug Administration (FDA) granted market clearance for the Xience Prime everolimus ...
November 1, 2011 – Boston Scientific released the schedule of its major events and product-related clinical research for ...

 November 15, 2011
November 15, 2011