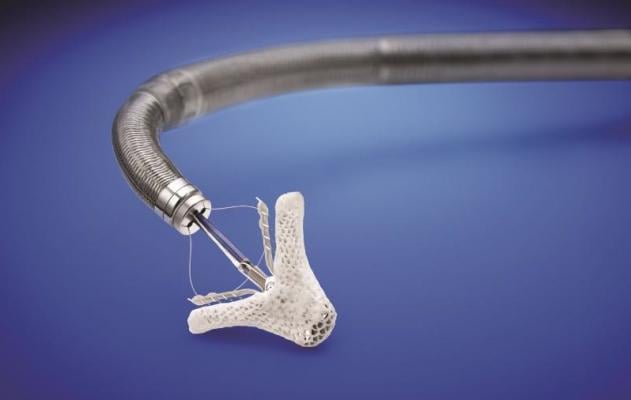
August 5, 2015 — Abbott announced Monday that it has entered into an agreement to purchase Tendyne Holdings Inc., a private medical device company focused on developing minimally invasive mitral valve replacement therapies. Under the terms of the agreement, Abbott will acquire the equity of Tendyne that it does not already own for $225 million upfront, resulting in a total transaction value of $250 million, plus potential future payments tied to regulatory milestones.
Tendyne's Bioprosthetic Mitral Valve System is an investigational device and not currently available for sale. The U.S. Food and Drug Administration has provided approval for a feasibility clinical trial to provide data about the device's safety and effectiveness. The trial has begun enrolling patients, and there are plans to begin enrollment next year in a clinical trial to support CE Mark in Europe.
In a separate transaction, Abbott has provided capital and secured an option to purchase Cephea Valve Technologies. Cephea Valve Technologies, a private company, is developing a catheter-based mitral valve replacement therapy. Financial terms were not disclosed.
As part of its structural heart portfolio, Abbott currently offers MitraClip, a mitral valve repair device used to treat certain types of mitral valve disease, the most prevalent heart valve disease. It is delivered to the heart via a catheter through the femoral vein. MitraClip is available in the United States, Canada, Europe and countries in Asia and Latin America.
The Tendyne and Cephea valve replacement technologies are designed to be implanted in a beating heart, without the need for open heart surgery, similar to MitraClip, thereby offering a new treatment option. Minimally invasive mitral valve repair and replacement is expected to become a multi-billion dollar market over the next 10 years.
Completion of the Tendyne acquisition is subject to customary closing conditions, including antitrust clearance. It is expected to close in the third quarter of this year. Neither the Tendyne acquisition nor the option to purchase Cephea is expected to impact Abbott's ongoing full-year 2015 earnings-per-share guidance.
Tendyne is headquartered in Roseville, Minnesota, and Cephea is headquartered in Santa Cruz, California.
For more information: www.abbottvascular.com


 July 08, 2024
July 08, 2024 









