May 2, 2017 — Acutus Medical recently announced that enrollment has been completed in its pivotal Utilizing Novel Dipole Density Capabilities to Objectively Visualize the Etiology of Rhythms in Atrial Fibrillation (UNCOVER AF) study. The study is evaluating the AcQMap High Resolution Imaging and Mapping System in patients with persistent atrial fibrillation (AF) in Europe and Canada.
The UNCOVER AF clinical study is a prospective, multi-center, multi-national study designed to provide clinical evidence regarding the use of the AcQMap System in the treatment of persistent AF. The trial is being conducted at 13 sites throughout Europe and Canada and has successfully completed enrollment of 129 patients.
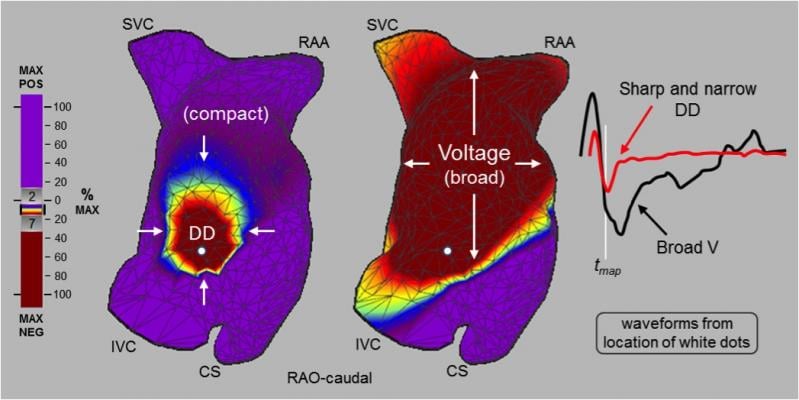
The technology reveals the electrical conduction of the whole heart chamber along with the heart wall motion (functional structure). The AcQMap System utilizes the combination of ultrasound to create a computed tomography (CT)-quality anatomy and non-contact, dipole density propagation maps to visualize previously unmappable complex arrhythmias (AF, Atypical Flutter) without the confounding effects of far field signals. This allows physicians for the first time to see real-time electrical activity within the heart chamber, with the ability to remap in seconds to confirm successful substrate modification via ablation therapy.
Acutus received CE Mark approval for AcQMap in May 2016 and is currently pursuing U.S. Food and Drug Administration (FDA) clearance for the system.
Additionally, the company is developing a significantly differentiated ablation technology designed to provide functionality that will further advance and support an electrophysiologist’s desire to produce improved, durable, patient-specific benefits.
For more information: www.acutusmedical.com


 December 19, 2025
December 19, 2025 









