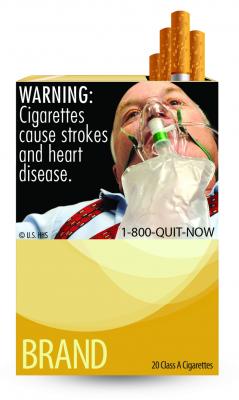
June 24, 2011 — American Heart Association (AHA) CEO Nancy Brown praised the new, larger tobacco packaging warning labels unveiled this week by the U.S. Food and Drug Administration (FDA). The labels include graphic images of disease caused by tobacco.
“New cigarette warning labels unveiled today by the Food and Drug Administration will help give us the momentum needed to eradicate tobacco use in our nation,” Brown said. “For the first time in 25 years, cigarette warning labels have been dramatically altered to graphically demonstrate the specific, serious health risks associated with smoking, and these warnings will not only tell smokers how bad tobacco use is, but also direct them to smoking cessation resources that can help them quit.”
According to the Centers for Disease Control and Prevention, more than 45 million Americans smoke cigarettes - about 20 percent of the population - and one in five high school students still smoke. The new health warnings represent an aggressive approach to reducing smoking rates that have leveled off in recent years as tobacco companies continue to launch campaigns to entice new smokers and maintain current customers.
“The American Heart Association strongly believes that the graphic depictions of smoking-related diseases on cigarette packages will drive home the message that tobacco use is an equal opportunity killer, affecting smokers and nonsmokers alike,” Brown said. “In the U.S., about one-third of smoking-related deaths are linked to heart disease and stroke. Cigarette smoking causes about 443,000 premature deaths each year and about 49,000 of these deaths are due to second-hand smoke. Undoubtedly, the new graphic health warnings will heighten awareness about the dangers of smoking and, more importantly, encourage smokers to quit and discourage smoking initiation. We're confident that the new labels will move us closer to our goal of making the nation 100 percent smoke-free.”
For more information: www.heart.org


 January 05, 2026
January 05, 2026 









