February 9, 2011 – The U.S. Food and Drug Administration (FDA) said certain lots of the Arstasis One femoral artery access system are part of a Class 1 recall. Components of the device may fracture and/or separate during use, which may result in patient harm, the FDA said.
The models included in the recall are the AAD100 and AAD101, part numbers FG-02279 and FG-03010, and lot numbers 09I10268, 1OC26337, 09J06281 and 10C12334.
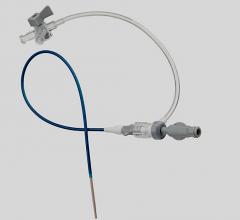
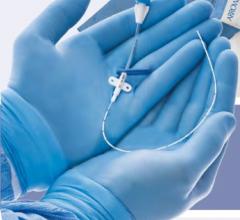
The system consists of the following components: Device sheath/anchor, shaft and a handle with control features. These products were distributed from May 14, 2010 through Oct. 13, 2010.
The Arstasis One Access System is used in patients undergoing diagnostic femoral artery catheterization procedures. It provides device access into the vascular system. This product also helps to stop the artery from bleeding when used in conjunction with manual compression.
The company initiated the recall Oct. 19, 2010. The company sent its customers a recall letter informing them that they are improving their product. The company will replace all existing devices with the improved product in their customers’ inventory that has not yet been used.
Customers were instructed to work closely with their local Arstasis territory manager to ensure that their product replacement is handled satisfactorily. Arstasis Customer Service can be contacted 877.688.8417, or by e-mail at [email protected].

 June 10, 2020
June 10, 2020