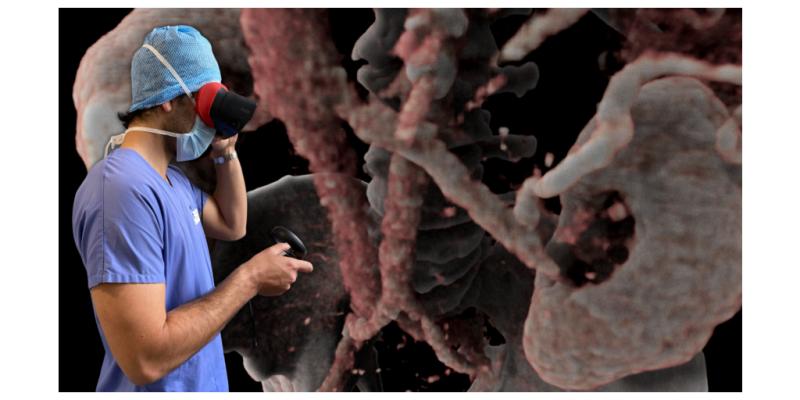
Surgeon visualizing a kidney cancer CTScan to plan his partial nephrectomy. (Photo: Business Wire)
June 12, 2023 — Medtech startup Avatar Medical announced today that its virtual reality (VR) surgical planning solution has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
Avatar Medical helps surgeons better prepare their procedures through the use of virtual reality (VR) representations of their patients. These patient avatars are generated instantly from CT scan or MRI images with an underlying proprietary technology based on four years of research in human-data interaction and machine learning conducted at the Institut Pasteur and Institut Curie. They serve as valuable tools for pre-operative planning and can also be displayed during surgical procedures.
More than 100 surgeons from 20 different hospitals and universities, including UMass, CUNY and Columbia University, have benefited from the solution. To date, it has been used for case studies, student education and patient engagement, leading to 6 medical publications.
“Avatar Medical is a unique hardware agnostic XR software platform. With the ability to plan based on preoperative imaging and then evaluate as well as confirm based on intraoperative imaging, I now have an additional level of capability and confidence in my advanced percutaneous and endovascular interventions.” Says Venkatesh Krishnasamy MD, Interventional Radiology, Associate Professor of Radiology, Columbia University.
“After receiving tremendous positive feedback from surgeons over the past 3 years, the FDA’s clearance is a major milestone in getting our VR solution in their hands to care for their patients. We expect European medical device certification for next year”, says Xavier Wartelle, CEO of Avatar Medical.
For more information: https://avatarmedical.ai/


 February 02, 2026
February 02, 2026 









